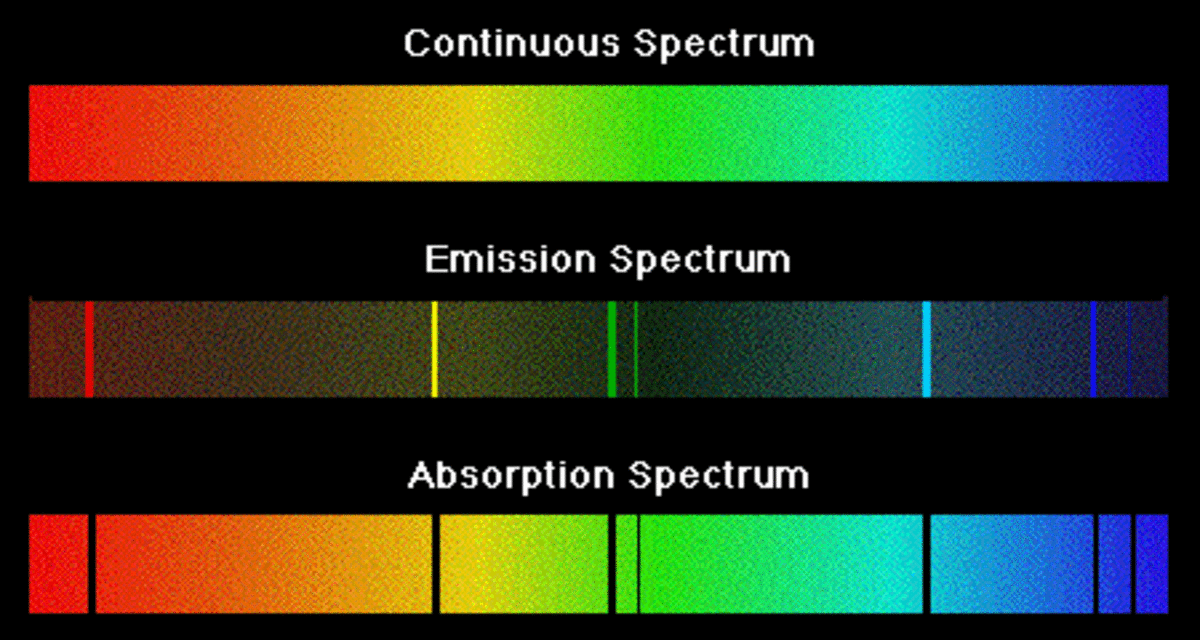
Emission Spectra Youtube . When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or. Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas. From earth, we can figure out the chemical composition of a star looking at the absorption spectrum. The line spectrum has certain, fixed. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. This video is a discussion about emission spectra and the bohr model, two very important concepts which dramatically.
from discover.hubpages.com
The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The line spectrum has certain, fixed. Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas. From earth, we can figure out the chemical composition of a star looking at the absorption spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. This video is a discussion about emission spectra and the bohr model, two very important concepts which dramatically. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra.
What Is The Difference Between Emission Spectra and Absorption Spectra
Emission Spectra Youtube When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. This video is a discussion about emission spectra and the bohr model, two very important concepts which dramatically. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or. The line spectrum has certain, fixed. From earth, we can figure out the chemical composition of a star looking at the absorption spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas.
From www.youtube.com
Bright LIne Emission Spectrum of Water Vapor YouTube Emission Spectra Youtube For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas. For higher physics, revise emission or absorption of certain frequencies of light from the elements and. Emission Spectra Youtube.
From www.youtube.com
2.2 Hydrogen emission spectrum (SL) YouTube Emission Spectra Youtube For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or. The line spectrum has certain, fixed. From earth, we can figure out the chemical composition of. Emission Spectra Youtube.
From www.youtube.com
ICPAESInductively coupled plasmaAtomic emission spectroscopy Emission Spectra Youtube From earth, we can figure out the chemical composition of a star looking at the absorption spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted. Emission Spectra Youtube.
From www.youtube.com
Atomic Emission Spectra Lab YouTube Emission Spectra Youtube Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas. From earth, we can figure out the chemical composition of a star looking at the absorption spectrum. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line. Emission Spectra Youtube.
From www.youtube.com
Unit 7 Emission Spectra YouTube Emission Spectra Youtube For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas. The line spectrum has certain, fixed. For higher physics, revise emission or absorption of certain frequencies. Emission Spectra Youtube.
From www.edinst.com
What are Absorption, Excitation and Emission Spectra? Emission Spectra Youtube When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide. Emission Spectra Youtube.
From www.youtube.com
Absorption spectrum and Emission spectrum video YouTube Emission Spectra Youtube This video is a discussion about emission spectra and the bohr model, two very important concepts which dramatically. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of. Emission Spectra Youtube.
From www.youtube.com
Emission and absorption spectra YouTube Emission Spectra Youtube For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. From earth, we can figure out the chemical composition of a star looking at the absorption spectrum. This video is. Emission Spectra Youtube.
From www.youtube.com
Xenon lamp spectrum YouTube Emission Spectra Youtube For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. From earth, we can figure out the chemical composition of a star looking at the absorption spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. Emission Spectra Youtube.
From discover.hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Emission Spectra Youtube When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The line spectrum has certain, fixed. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or. For higher physics, revise emission or absorption of. Emission Spectra Youtube.
From www.youtube.com
Emission and Absorption Line Spectra A Level Physics YouTube Emission Spectra Youtube For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element. Emission Spectra Youtube.
From www.youtube.com
03.03 The Emission Spectrum of the Hydrogen Atom YouTube Emission Spectra Youtube From earth, we can figure out the chemical composition of a star looking at the absorption spectrum. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or. Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete. Emission Spectra Youtube.
From www.youtube.com
Emission and Absorption Spectra YouTube Emission Spectra Youtube Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or. Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas. This video is a discussion about emission spectra and the. Emission Spectra Youtube.
From www.youtube.com
Absorption and Emission Spectra YouTube Emission Spectra Youtube Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or. When hydrogen gas is placed into a tube and electric. Emission Spectra Youtube.
From www.youtube.com
Difference Between Absorption and Emission Spectra YouTube Emission Spectra Youtube This video is a discussion about emission spectra and the bohr model, two very important concepts which dramatically. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or. From earth, we can figure out the chemical composition of a star looking at the absorption spectrum.. Emission Spectra Youtube.
From www.youtube.com
Emissions Spectra YouTube Emission Spectra Youtube The line spectrum has certain, fixed. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. For higher physics, revise emission or absorption of. Emission Spectra Youtube.
From www.youtube.com
Atoms Class 12 Physics Atomic Spectra YouTube Emission Spectra Youtube The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. This video is a discussion about emission spectra and the bohr model, two. Emission Spectra Youtube.
From www.youtube.com
Atomic spectra , Emission and absorption spectra Hydrogen spectra and Emission Spectra Youtube When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. This video is a discussion about emission spectra and the bohr model, two very important concepts which dramatically. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. The line. Emission Spectra Youtube.
From www.youtube.com
Emission and absorption spectra YouTube Emission Spectra Youtube The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or. This video is a discussion about emission spectra and. Emission Spectra Youtube.
From www.youtube.com
Deconvolution of photoluminescence PL spectra (peaks fitting) 22 Emission Spectra Youtube The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The line spectrum has certain, fixed. This video is a discussion about emission spectra and the bohr model, two very important concepts which dramatically. For higher physics, revise emission or absorption of certain frequencies. Emission Spectra Youtube.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Emission Spectra Youtube This video is a discussion about emission spectra and the bohr model, two very important concepts which dramatically. Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas. When hydrogen gas is placed into a tube and electric current passed through it, the color of. Emission Spectra Youtube.
From www.youtube.com
CONTINUOUS SPECTRUM YouTube Emission Spectra Youtube For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed into a tube and electric current passed through it, the. Emission Spectra Youtube.
From www.youtube.com
Emission spectrum of hydrogen Chemistry Khan Academy YouTube Emission Spectra Youtube When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or. For higher physics, revise emission or absorption of certain frequencies of light from the. Emission Spectra Youtube.
From www.youtube.com
Emission Spectrum Interstellar Station YouTube Emission Spectra Youtube The line spectrum has certain, fixed. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Hot gases produce emission line spectra when photons are. Emission Spectra Youtube.
From www.youtube.com
Flame photometry/Flame Emission Spectroscopy (FES)/Atomic emission Emission Spectra Youtube Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas. The line spectrum has certain, fixed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. For higher physics,. Emission Spectra Youtube.
From www.youtube.com
Bright Line Emission Spectrum of Air YouTube Emission Spectra Youtube Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. This video is a discussion about emission spectra and the bohr model, two very important concepts. Emission Spectra Youtube.
From www.youtube.com
C3 Absorption, Line, Emission and Continuous Spectra [SL IB Chemistry Emission Spectra Youtube Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas. Emission and absorption spectra form the basis of spectroscopy, which uses spectra to provide information about the structure and the composition of a substance or. The emission spectrum (or line spectrum) of a chemical element. Emission Spectra Youtube.
From www.youtube.com
Electron excitation, emission and absorption spectra YouTube Emission Spectra Youtube Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. This video is a discussion about emission spectra and the bohr model, two very important concepts. Emission Spectra Youtube.
From www.youtube.com
Bright Line Emission Spectrum of Nitrogen YouTube Emission Spectra Youtube The line spectrum has certain, fixed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Hot gases produce emission line spectra when. Emission Spectra Youtube.
From www.youtube.com
HYDROGEN SPECTRUM in english YouTube Emission Spectra Youtube When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The line spectrum has certain, fixed. This video is a discussion about emission spectra and the bohr model, two very important concepts which dramatically. For higher physics, revise emission or absorption of certain frequencies of light from the elements. Emission Spectra Youtube.
From www.youtube.com
2emission and absorption spectra YouTube Emission Spectra Youtube For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. This video is a discussion about emission spectra and the bohr model, two very important concepts which dramatically. The emission. Emission Spectra Youtube.
From www.youtube.com
5.3 Atomic Emission Spectra & the Quantum Mechanical Model YouTube Emission Spectra Youtube When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas. This video is a discussion about emission spectra and the bohr model, two very. Emission Spectra Youtube.
From www.youtube.com
2.3.2 Distinguish between a continuous spectrum and a line spectrum Emission Spectra Youtube Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. For higher physics, revise emission or absorption of certain frequencies of light from the elements and. Emission Spectra Youtube.
From www.edinst.com
TimeResolved Emission Spectroscopy Sb to Mn Energy Transfer Emission Spectra Youtube This video is a discussion about emission spectra and the bohr model, two very important concepts which dramatically. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line spectra. From earth,. Emission Spectra Youtube.
From www.youtube.com
Atomic Emission Spectroscopy YouTube Emission Spectra Youtube From earth, we can figure out the chemical composition of a star looking at the absorption spectrum. Hot gases produce emission line spectra when photons are emitted due to the transition of electrons between discrete energy levels in atoms of the gas. For higher physics, revise emission or absorption of certain frequencies of light from the elements and atomic line. Emission Spectra Youtube.