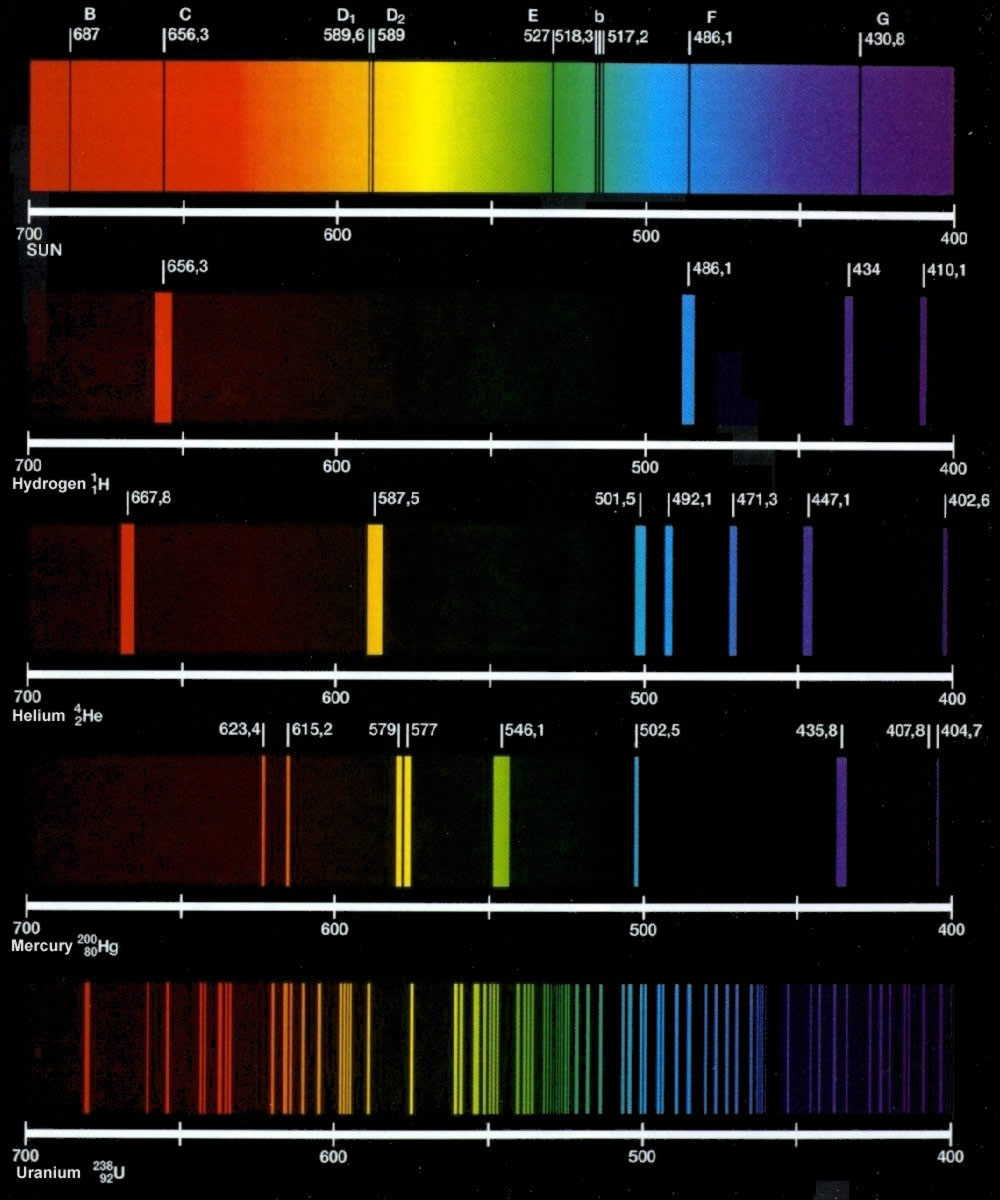
What Is The Emission Spectrum Of Hydrogen . This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. When the emitted light is passed through a prism, only a few narrow lines, called a line spectrum, which is a spectrum in which light of only a. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. 10 lines as being emissions of atomic hydrogen. When this light is passed. We all know that electrons in an atom or a molecule. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical.
from penrodkur.blogspot.com
10 lines as being emissions of atomic hydrogen. When this light is passed. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. When the emitted light is passed through a prism, only a few narrow lines, called a line spectrum, which is a spectrum in which light of only a. We all know that electrons in an atom or a molecule.
Line Spectrum Of Hydrogen Modelo Atómico de Bohr Características y
What Is The Emission Spectrum Of Hydrogen This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. We all know that electrons in an atom or a molecule. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. 10 lines as being emissions of atomic hydrogen. When this light is passed. When the emitted light is passed through a prism, only a few narrow lines, called a line spectrum, which is a spectrum in which light of only a. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels.
From chem.libretexts.org
7.3 The Atomic Spectrum of Hydrogen Chemistry LibreTexts What Is The Emission Spectrum Of Hydrogen 10 lines as being emissions of atomic hydrogen. We all know that electrons in an atom or a molecule. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. When the emitted light. What Is The Emission Spectrum Of Hydrogen.
From axeljoysoconnor.blogspot.com
Atomic Emission Spectrum of Hydrogen AxeljoysOconnor What Is The Emission Spectrum Of Hydrogen When this light is passed. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. We all know that electrons in an atom or a molecule. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. When the emitted. What Is The Emission Spectrum Of Hydrogen.
From www.youtube.com
Emission spectrum of hydrogen Chemistry Khan Academy YouTube What Is The Emission Spectrum Of Hydrogen 10 lines as being emissions of atomic hydrogen. When the emitted light is passed through a prism, only a few narrow lines, called a line spectrum, which is a spectrum in which light of only a. When this light is passed. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. We. What Is The Emission Spectrum Of Hydrogen.
From byjus.com
Spectral Series Explained along with Hydrogen spectrum, Rydberg formula. What Is The Emission Spectrum Of Hydrogen When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. We all know that electrons. What Is The Emission Spectrum Of Hydrogen.
From chemwiki.ucdavis.edu
The Bohr Atom Chemwiki What Is The Emission Spectrum Of Hydrogen 10 lines as being emissions of atomic hydrogen. We all know that electrons in an atom or a molecule. When the emitted light is passed through a prism, only a few narrow lines, called a line spectrum, which is a spectrum in which light of only a. This page introduces the atomic hydrogen emission spectrum, showing how it arises from. What Is The Emission Spectrum Of Hydrogen.
From byjus.com
Hydrogen Spectrum Balmer Series, Definition, Diagram, Spectrum What Is The Emission Spectrum Of Hydrogen This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. We all know that electrons in an atom or a molecule. It may seem surprising that lines of the hydrogen spectrum were seen in. What Is The Emission Spectrum Of Hydrogen.
From www.youtube.com
The Emission Spectrum of the Hydrogen Atom YouTube What Is The Emission Spectrum Of Hydrogen 10 lines as being emissions of atomic hydrogen. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements. What Is The Emission Spectrum Of Hydrogen.
From mavink.com
Absorption Spectrum Of Hydrogen What Is The Emission Spectrum Of Hydrogen We all know that electrons in an atom or a molecule. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. When this light is passed. When the emitted light is passed through a prism, only a few. What Is The Emission Spectrum Of Hydrogen.
From www.vrogue.co
The Emission Spectrum Of The Hydrogen Atom Youtube vrogue.co What Is The Emission Spectrum Of Hydrogen 10 lines as being emissions of atomic hydrogen. When this light is passed. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. When the emitted light is passed through a prism, only a few narrow lines, called a line spectrum, which is a spectrum in which light of only a. We all know that electrons. What Is The Emission Spectrum Of Hydrogen.
From ece.umd.edu
Hydrogen atom, spectrum and electron transitions What Is The Emission Spectrum Of Hydrogen When this light is passed. 10 lines as being emissions of atomic hydrogen. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. When an electric current is passed through a glass tube that contains hydrogen gas at. What Is The Emission Spectrum Of Hydrogen.
From pressbooks.online.ucf.edu
30.3 Bohr’s Theory of the Hydrogen Atom College Physics What Is The Emission Spectrum Of Hydrogen We all know that electrons in an atom or a molecule. When the emitted light is passed through a prism, only a few narrow lines, called a line spectrum, which is a spectrum in which light of only a. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off. What Is The Emission Spectrum Of Hydrogen.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts What Is The Emission Spectrum Of Hydrogen It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. When the emitted light is passed through a prism, only a few narrow lines, called a line spectrum, which is a spectrum in which light of only a.. What Is The Emission Spectrum Of Hydrogen.
From emiliano-blogmassey.blogspot.com
Describe the Emission Spectrum of Hydrogen Ib Chemistry What Is The Emission Spectrum Of Hydrogen When this light is passed. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light.. What Is The Emission Spectrum Of Hydrogen.
From chem.libretexts.org
7.3 The Atomic Spectrum of Hydrogen Chemistry LibreTexts What Is The Emission Spectrum Of Hydrogen This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. We all know that electrons in an atom or a molecule. 10 lines as being emissions of atomic hydrogen. This page introduces the atomic hydrogen emission spectrum, showing. What Is The Emission Spectrum Of Hydrogen.
From ubicaciondepersonas.cdmx.gob.mx
Emission Spectrum Of Hydrogen ubicaciondepersonas.cdmx.gob.mx What Is The Emission Spectrum Of Hydrogen When the emitted light is passed through a prism, only a few narrow lines, called a line spectrum, which is a spectrum in which light of only a. 10 lines as being emissions of atomic hydrogen. When this light is passed. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube. What Is The Emission Spectrum Of Hydrogen.
From www.youtube.com
HYDROGEN SPECTRUM in english YouTube What Is The Emission Spectrum Of Hydrogen We all know that electrons in an atom or a molecule. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. 10 lines as being emissions of atomic hydrogen. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. When an electric current is passed through a glass. What Is The Emission Spectrum Of Hydrogen.
From www.slideserve.com
PPT The color of the light emitted depends upon the D E as the What Is The Emission Spectrum Of Hydrogen When the emitted light is passed through a prism, only a few narrow lines, called a line spectrum, which is a spectrum in which light of only a. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. When this light is passed. This page introduces the atomic hydrogen emission spectrum, showing. What Is The Emission Spectrum Of Hydrogen.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps What Is The Emission Spectrum Of Hydrogen We all know that electrons in an atom or a molecule. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. When this light is passed. 10 lines as being emissions of atomic. What Is The Emission Spectrum Of Hydrogen.
From penrodkur.blogspot.com
Line Spectrum Of Hydrogen Modelo Atómico de Bohr Características y What Is The Emission Spectrum Of Hydrogen We all know that electrons in an atom or a molecule. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. When the emitted light is passed through a prism, only a few narrow lines, called a line spectrum, which is a spectrum in which light of. What Is The Emission Spectrum Of Hydrogen.
From classnotes.org.in
Absorption and Emission Spectra Chemistry, Class 11, Structure Of Atom What Is The Emission Spectrum Of Hydrogen When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. When this light is passed.. What Is The Emission Spectrum Of Hydrogen.
From classnotes.org.in
Hydrogen spectrum Chemistry, Class 11, Structure Of Atom What Is The Emission Spectrum Of Hydrogen 10 lines as being emissions of atomic hydrogen. We all know that electrons in an atom or a molecule. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light.. What Is The Emission Spectrum Of Hydrogen.
From chem.libretexts.org
11.4 Bohr's Theory of the Hydrogen Emission Spectrum Chemistry What Is The Emission Spectrum Of Hydrogen This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. 10 lines as being emissions of atomic hydrogen. We all know that electrons in an atom or a molecule.. What Is The Emission Spectrum Of Hydrogen.
From mavink.com
Visible Line Spectrum Of Hydrogen What Is The Emission Spectrum Of Hydrogen When the emitted light is passed through a prism, only a few narrow lines, called a line spectrum, which is a spectrum in which light of only a. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. When this light is passed. We all know that electrons in an atom or. What Is The Emission Spectrum Of Hydrogen.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum What Is The Emission Spectrum Of Hydrogen When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. We all know that electrons in an atom or a molecule. When the emitted light is passed through a prism, only a few narrow lines, called a line spectrum, which is a spectrum in which light of. What Is The Emission Spectrum Of Hydrogen.
From byjus.com
Hydrogen Spectrum Balmer SeriesDefinitionDiagram Chemistry Byju’s What Is The Emission Spectrum Of Hydrogen We all know that electrons in an atom or a molecule. When this light is passed. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. 10 lines as being emissions of atomic hydrogen. When the emitted light. What Is The Emission Spectrum Of Hydrogen.
From www.slideserve.com
PPT Electrons and Periodicity PowerPoint Presentation, free download What Is The Emission Spectrum Of Hydrogen 10 lines as being emissions of atomic hydrogen. We all know that electrons in an atom or a molecule. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. When this light is passed. This page introduces the atomic hydrogen emission spectrum, showing how it arises from. What Is The Emission Spectrum Of Hydrogen.
From www.sciencephoto.com
Hydrogen emission and absorption spectra Stock Image C025/8082 What Is The Emission Spectrum Of Hydrogen When this light is passed. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. When the emitted light is. What Is The Emission Spectrum Of Hydrogen.
From www.slideserve.com
PPT Atomic Emission Spectra PowerPoint Presentation, free download What Is The Emission Spectrum Of Hydrogen It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. 10 lines as being emissions of atomic hydrogen. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. When an. What Is The Emission Spectrum Of Hydrogen.
From dotgola.weebly.com
Hydrogen line emission spectrum dotgola What Is The Emission Spectrum Of Hydrogen When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. When the emitted light is passed through a prism, only a few narrow lines, called a line spectrum, which is a spectrum in which light of only a. It may seem surprising that lines of the hydrogen. What Is The Emission Spectrum Of Hydrogen.
From winstonmcyponce.blogspot.com
Atomic Emission Spectrum of Hydrogen WinstonmcyPonce What Is The Emission Spectrum Of Hydrogen This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. This page introduces the atomic. What Is The Emission Spectrum Of Hydrogen.
From mungfali.com
Hydrogen Atomic Emission Spectrum What Is The Emission Spectrum Of Hydrogen We all know that electrons in an atom or a molecule. When this light is passed. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. When the emitted light is passed through a prism, only a few. What Is The Emission Spectrum Of Hydrogen.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra What Is The Emission Spectrum Of Hydrogen When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. 10 lines as being emissions of atomic hydrogen. When the emitted light is passed through a prism, only a few narrow lines, called a line spectrum, which is a spectrum in which light of only a. When. What Is The Emission Spectrum Of Hydrogen.
From www.youtube.com
2.2 Hydrogen emission spectrum (SL) YouTube What Is The Emission Spectrum Of Hydrogen This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. 10 lines as being emissions of atomic hydrogen. We all know that electrons in an atom or a molecule. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light.. What Is The Emission Spectrum Of Hydrogen.
From www.vernier.com
Spectrum of Atomic Hydrogen > Experiment 21 from Advanced Physics with What Is The Emission Spectrum Of Hydrogen 10 lines as being emissions of atomic hydrogen. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light. It may seem surprising that lines of the hydrogen spectrum were seen in astronomical. When the emitted light is passed through a prism, only a few narrow lines, called. What Is The Emission Spectrum Of Hydrogen.
From srkjpxyrfzubc.blogspot.com
Helium Emission Spectrum, Hydrogen Fusion and the Emission Spectrum A What Is The Emission Spectrum Of Hydrogen When the emitted light is passed through a prism, only a few narrow lines, called a line spectrum, which is a spectrum in which light of only a. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels. We all know that electrons in an atom or a molecule. When an electric. What Is The Emission Spectrum Of Hydrogen.