How Many Grams Of Iron Are Produced . How many grams of iron is produced when 25.0 mol. If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na to determine how many moles of \(nacl\). Iron is obtained commercially by the reaction of hematite #fe_2o_3# with carbon monoxide. Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron can be produced? Question asks about grams of fe 2 o 3;
from www.bartleby.com
How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? How many grams of iron is produced when 25.0 mol. If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na to determine how many moles of \(nacl\). If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron can be produced? Iron is obtained commercially by the reaction of hematite #fe_2o_3# with carbon monoxide. Question asks about grams of fe 2 o 3; Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration.
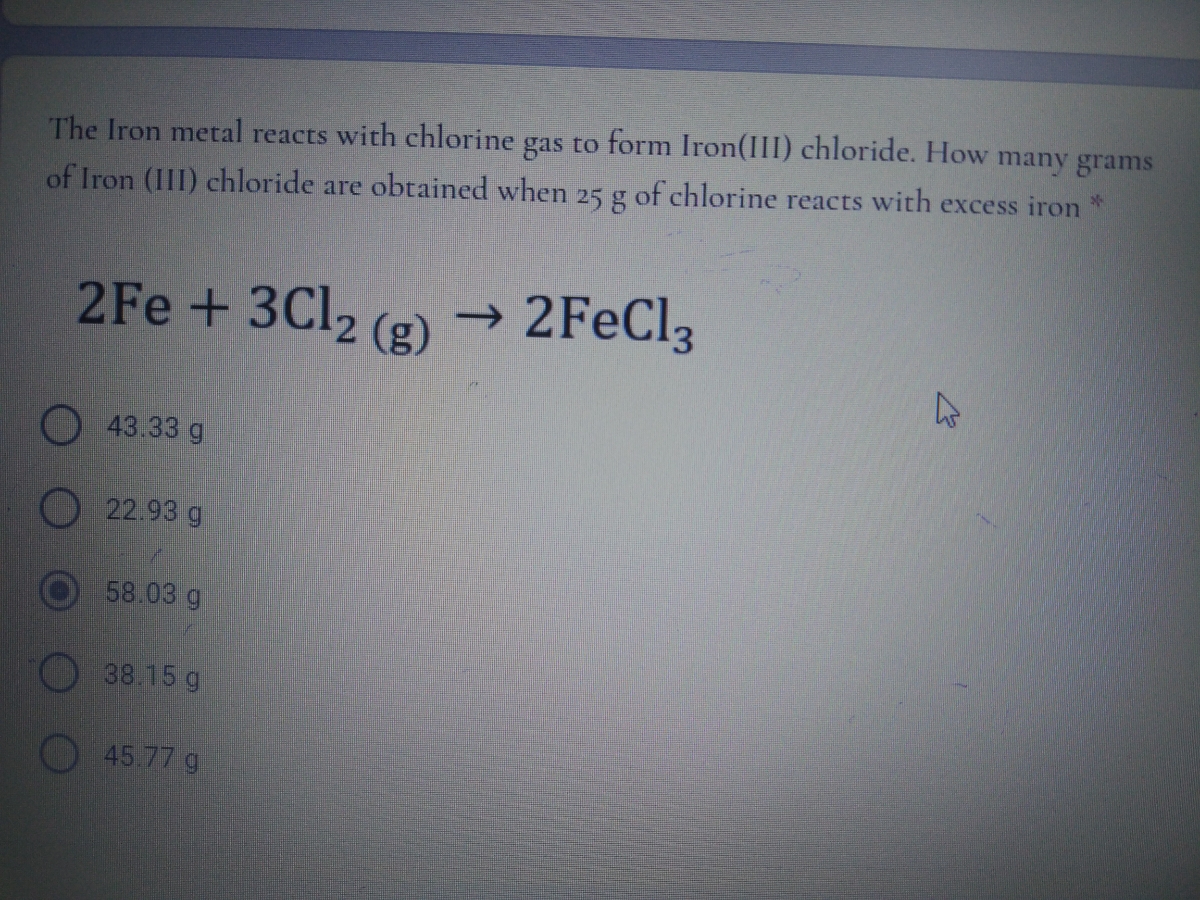
Answered The Iron metal reacts with chlorine gas… bartleby
How Many Grams Of Iron Are Produced How many grams of iron is produced when 25.0 mol. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron can be produced? Iron is obtained commercially by the reaction of hematite #fe_2o_3# with carbon monoxide. If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na to determine how many moles of \(nacl\). How many grams of iron is produced when 25.0 mol. Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. Question asks about grams of fe 2 o 3;
From www.slideserve.com
PPT Chapter 6 Chemical Composition Counting Atoms PowerPoint How Many Grams Of Iron Are Produced Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. Question asks about grams of fe 2 o 3; Iron is obtained commercially by the reaction of hematite #fe_2o_3# with carbon monoxide. How many grams of iron is produced when 25.0 mol. How many grams fe 2 o 3, are produced from the. How Many Grams Of Iron Are Produced.
From joiuzqgmu.blob.core.windows.net
How Much Is Iron Per Gram at Michelle Tharp blog How Many Grams Of Iron Are Produced If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron can be produced? How many grams of iron is produced when 25.0 mol. Iron is obtained commercially by the reaction of hematite #fe_2o_3# with carbon monoxide. If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of. How Many Grams Of Iron Are Produced.
From www.chegg.com
Solved How many grams of iron are needed to produce 19.1 L How Many Grams Of Iron Are Produced Question asks about grams of fe 2 o 3; How many grams of iron is produced when 25.0 mol. Iron is obtained commercially by the reaction of hematite #fe_2o_3# with carbon monoxide. Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. How many grams fe 2 o 3, are produced from the. How Many Grams Of Iron Are Produced.
From www.chegg.com
Solved How many grams of iron must be oxidized to produce How Many Grams Of Iron Are Produced Question asks about grams of fe 2 o 3; Iron is obtained commercially by the reaction of hematite #fe_2o_3# with carbon monoxide. How many grams of iron is produced when 25.0 mol. Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. If 25.0 kilograms of pure fe 2 o 3 is used,. How Many Grams Of Iron Are Produced.
From www.bartleby.com
Answered The Iron metal reacts with chlorine gas… bartleby How Many Grams Of Iron Are Produced Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. How many grams of iron is produced when 25.0 mol. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? Question asks about grams of fe 2 o 3; Iron is obtained commercially by the. How Many Grams Of Iron Are Produced.
From brainly.com
How many grams of iron (III) oxide are produced when 38 grams of How Many Grams Of Iron Are Produced Question asks about grams of fe 2 o 3; If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na to determine how many moles of \(nacl\). Iron is obtained commercially by the reaction of hematite #fe_2o_3# with carbon monoxide. How many grams fe 2 o 3,. How Many Grams Of Iron Are Produced.
From www.chegg.com
Solved How many grams of iron are needed to completely How Many Grams Of Iron Are Produced How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron can be produced? If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of. How Many Grams Of Iron Are Produced.
From www.numerade.com
SOLVED 1. Iron reacts with oxygen to produce iron(III)oxide =. The How Many Grams Of Iron Are Produced Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. Question asks about grams of fe 2 o 3; If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na to determine how many moles of \(nacl\). How many grams. How Many Grams Of Iron Are Produced.
From brainly.com
1. How many grams of iron (II) oxide can be produced from 3.4 g of iron How Many Grams Of Iron Are Produced If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron can be produced? How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? How many grams of iron is produced when 25.0 mol. Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of. How Many Grams Of Iron Are Produced.
From www.slideserve.com
PPT 1 mole = 6.02 X 10 23 things This is called Avogadro’s number How Many Grams Of Iron Are Produced If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron can be produced? How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of. How Many Grams Of Iron Are Produced.
From www.numerade.com
SOLVEDHow many grams of water are required to dissolve 12.7 grams of How Many Grams Of Iron Are Produced Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na to determine how many moles of \(nacl\). Iron is obtained commercially by the reaction of hematite #fe_2o_3# with carbon. How Many Grams Of Iron Are Produced.
From www.chegg.com
Solved 3. Iron reacts with oxygen to form iron (III) oxide How Many Grams Of Iron Are Produced How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? Question asks about grams of fe 2 o 3; If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na to determine how many moles of \(nacl\). Iron is. How Many Grams Of Iron Are Produced.
From www.pinterest.com.au
This infographic shows 20 iron rich foods alongside the amount of iron How Many Grams Of Iron Are Produced Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. How many grams of iron is produced when 25.0 mol. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? Question asks about grams of fe 2 o 3; If we know how many moles. How Many Grams Of Iron Are Produced.
From www.chegg.com
Solved If 501J of heat is available, how many grams of iron How Many Grams Of Iron Are Produced If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron can be produced? If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na to determine how many moles of \(nacl\). How many grams fe 2 o 3, are produced from. How Many Grams Of Iron Are Produced.
From www.coursehero.com
[Solved] Exercise 9.34 V Part A How many grams of iron(III) carbonate How Many Grams Of Iron Are Produced If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na to determine how many moles of \(nacl\). How many grams of iron is produced when 25.0 mol. If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron can be produced?. How Many Grams Of Iron Are Produced.
From www.chegg.com
Solved 5. Calculate how many grams of iron can be made from How Many Grams Of Iron Are Produced Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. How many grams of iron is produced when 25.0 mol. If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron can be produced? Iron is obtained commercially by the reaction of hematite #fe_2o_3# with carbon monoxide. Question. How Many Grams Of Iron Are Produced.
From www.numerade.com
SOLVED 15.0 grams of Iron is allowed to react with air to produce Iron How Many Grams Of Iron Are Produced How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron can be produced? Question asks about grams of fe 2 o 3; If we know how many moles of \(na\) reacted, we can use the ratio. How Many Grams Of Iron Are Produced.
From www.coursehero.com
[Solved] II. How many grams of iron, Fe, are produced with 25 grams of How Many Grams Of Iron Are Produced Iron is obtained commercially by the reaction of hematite #fe_2o_3# with carbon monoxide. If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na to determine how many moles of \(nacl\). Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known. How Many Grams Of Iron Are Produced.
From exymxlkfq.blob.core.windows.net
How Many Grams Are In 1 Mole Of Iron at Gary Rencher blog How Many Grams Of Iron Are Produced Question asks about grams of fe 2 o 3; Iron is obtained commercially by the reaction of hematite #fe_2o_3# with carbon monoxide. How many grams of iron is produced when 25.0 mol. If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron can be produced? Quantitative calculations that involve the stoichiometry of reactions in. How Many Grams Of Iron Are Produced.
From www.coursehero.com
[Solved] 1) How many grams of iron metal will be deposited from a How Many Grams Of Iron Are Produced If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na to determine how many moles of \(nacl\). Iron is obtained commercially by the reaction of hematite #fe_2o_3# with carbon monoxide. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g. How Many Grams Of Iron Are Produced.
From www.coursehero.com
[Solved] 1) How many grams of iron metal will be deposited from a How Many Grams Of Iron Are Produced If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron can be produced? How many grams of iron is produced when 25.0 mol. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? Question asks about grams of fe 2 o 3; If we know how. How Many Grams Of Iron Are Produced.
From www.toppr.com
Calculate the number of moles in 112 g of iron. How Many Grams Of Iron Are Produced Question asks about grams of fe 2 o 3; Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron. How Many Grams Of Iron Are Produced.
From www.numerade.com
SOLVED How many grams of iron(III) sulfide form when 62.0 mL of 0.135 How Many Grams Of Iron Are Produced If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron can be produced? Question asks about grams of fe 2 o 3; Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. How many grams fe 2 o 3, are produced from the complete reaction of 32.2. How Many Grams Of Iron Are Produced.
From www.chegg.com
Solved Name ID A d. 135 g 19.) How many grams of iron(III) How Many Grams Of Iron Are Produced Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron can be produced? Question asks about grams of fe 2 o 3; If we know how many moles of \(na\) reacted, we can use the ratio of. How Many Grams Of Iron Are Produced.
From www.slideserve.com
PPT 9. 2 C 3 H 7 OH + 9 O 2 → 6 CO 2 + 8 H 2 O PowerPoint How Many Grams Of Iron Are Produced Iron is obtained commercially by the reaction of hematite #fe_2o_3# with carbon monoxide. If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na to determine how many moles of \(nacl\). How many grams of iron is produced when 25.0 mol. Quantitative calculations that involve the stoichiometry. How Many Grams Of Iron Are Produced.
From slideplayer.com
Unit 8 Reactions And Stoichiometry ppt download How Many Grams Of Iron Are Produced If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na to determine how many moles of \(nacl\). How many grams of iron is produced when 25.0 mol. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? If. How Many Grams Of Iron Are Produced.
From slideplayer.com
Stoichiometry II. ppt download How Many Grams Of Iron Are Produced Iron is obtained commercially by the reaction of hematite #fe_2o_3# with carbon monoxide. How many grams of iron is produced when 25.0 mol. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? Question asks about grams of fe 2 o 3; Quantitative calculations that involve the stoichiometry of reactions in solution. How Many Grams Of Iron Are Produced.
From studen.com
Will mark as brainliest ! how many grams of iron metal do you expect to How Many Grams Of Iron Are Produced How many grams of iron is produced when 25.0 mol. If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron can be produced? Question asks about grams of fe 2 o 3; If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles. How Many Grams Of Iron Are Produced.
From solvedlib.com
The thermite reaction, shown below,is used in welding… SolvedLib How Many Grams Of Iron Are Produced Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na to determine how many moles of \(nacl\). If 25.0 kilograms of pure fe 2 o 3 is used, how. How Many Grams Of Iron Are Produced.
From www.chegg.com
Solved STOICHIOMETRY PRACTICE SHOW ALL WORK11! 1. Iron How Many Grams Of Iron Are Produced How many grams of iron is produced when 25.0 mol. Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. Question asks about grams of fe 2 o 3; If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na. How Many Grams Of Iron Are Produced.
From www.coursehero.com
[Solved] 5. How many grams of iron will be dissolved by 23.4 g of How Many Grams Of Iron Are Produced Question asks about grams of fe 2 o 3; How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? Iron is obtained commercially by the reaction of hematite #fe_2o_3# with carbon monoxide. Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. If 25.0 kilograms. How Many Grams Of Iron Are Produced.
From brainly.com
How many grams of Fe2O3 will be produced from 37.5 grams of iron How Many Grams Of Iron Are Produced How many grams of iron is produced when 25.0 mol. If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na to determine how many moles of \(nacl\). Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. Iron is. How Many Grams Of Iron Are Produced.
From solvedlib.com
How many grams of iron (ii) iodide contain 4.60 grams… SolvedLib How Many Grams Of Iron Are Produced If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na to determine how many moles of \(nacl\). Quantitative calculations that involve the stoichiometry of reactions in solution use volumes of solutions of known concentration. If 25.0 kilograms of pure fe 2 o 3 is used, how. How Many Grams Of Iron Are Produced.
From www.numerade.com
SOLVED how many grams of iron will be required to release all the How Many Grams Of Iron Are Produced If we know how many moles of \(na\) reacted, we can use the ratio of 2 moles of \(nacl\) to 2 moles of na to determine how many moles of \(nacl\). Iron is obtained commercially by the reaction of hematite #fe_2o_3# with carbon monoxide. How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g. How Many Grams Of Iron Are Produced.
From slideplayer.com
Chapter 12 Stoichiometry 12.2 Chemical Calculations ppt download How Many Grams Of Iron Are Produced How many grams fe 2 o 3, are produced from the complete reaction of 32.2 g of fe? If 25.0 kilograms of pure fe 2 o 3 is used, how many kilograms of iron can be produced? Question asks about grams of fe 2 o 3; How many grams of iron is produced when 25.0 mol. Iron is obtained commercially. How Many Grams Of Iron Are Produced.