Is Q Heat Capacity . We now introduce two concepts useful in describing heat flow and temperature change. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). The symbol c stands for the specific heat. The heat \(q\) transferred to cause a temperature change depends on the magnitude of the temperature change, the mass of the system, and the substance and phase. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have. The heat cap acity (\(c\)) of a body of matter is the quantity of heat. Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta t\) is the change in temperature. The heat capacity of a defined object is usually expressed in joules or calories and temperature in kelvin or celsius. Where q is the energy added and δt is the change in temperature.
from studylib.net
The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have. The heat capacity of a defined object is usually expressed in joules or calories and temperature in kelvin or celsius. The heat \(q\) transferred to cause a temperature change depends on the magnitude of the temperature change, the mass of the system, and the substance and phase. Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta t\) is the change in temperature. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). The symbol c stands for the specific heat. We now introduce two concepts useful in describing heat flow and temperature change. The heat cap acity (\(c\)) of a body of matter is the quantity of heat. Where q is the energy added and δt is the change in temperature.
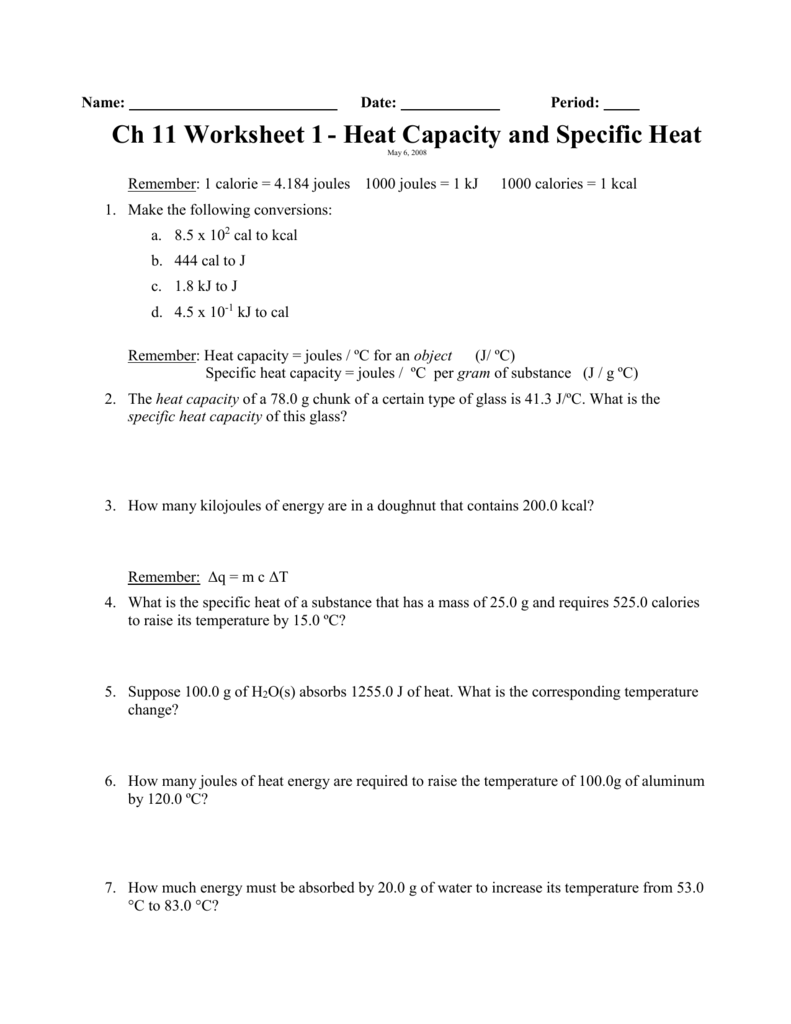
Heat Capacity and Specific Heat Worksheet 1 3/3/04 12641 PM
Is Q Heat Capacity We now introduce two concepts useful in describing heat flow and temperature change. The heat \(q\) transferred to cause a temperature change depends on the magnitude of the temperature change, the mass of the system, and the substance and phase. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have. Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta t\) is the change in temperature. The heat cap acity (\(c\)) of a body of matter is the quantity of heat. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Where q is the energy added and δt is the change in temperature. The heat capacity of a defined object is usually expressed in joules or calories and temperature in kelvin or celsius. The symbol c stands for the specific heat. We now introduce two concepts useful in describing heat flow and temperature change.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Is Q Heat Capacity Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta t\) is the change in temperature. Where q is the energy added and δt is the change in temperature. The heat cap acity (\(c\)) of a body of matter is the quantity of heat. We now introduce two concepts useful. Is Q Heat Capacity.
From www.studocu.com
Specific Heat Practice Problems Specific Heat Worksheet C = T/m ̈T Is Q Heat Capacity The heat capacity of a defined object is usually expressed in joules or calories and temperature in kelvin or celsius. We now introduce two concepts useful in describing heat flow and temperature change. Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta t\) is the change in temperature. The. Is Q Heat Capacity.
From haipernews.com
How To Calculate Heat Capacity From Enthalpy Haiper Is Q Heat Capacity The heat capacity of a defined object is usually expressed in joules or calories and temperature in kelvin or celsius. Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta t\) is the change in temperature. We now introduce two concepts useful in describing heat flow and temperature change. Where. Is Q Heat Capacity.
From getrevising.co.uk
Physicsend of year 9 Revision Cards in GCSE Physics Is Q Heat Capacity The heat cap acity (\(c\)) of a body of matter is the quantity of heat. Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta t\) is the change in temperature. The heat capacity of a defined object is usually expressed in joules or calories and temperature in kelvin or. Is Q Heat Capacity.
From www.slideserve.com
PPT Chapter10 Temperature and Heat PowerPoint Presentation, free Is Q Heat Capacity The symbol c stands for the specific heat. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have. The heat \(q\) transferred to cause a temperature change depends on the magnitude of the temperature change, the mass of the system, and the substance and. Is Q Heat Capacity.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Is Q Heat Capacity Where q is the energy added and δt is the change in temperature. Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta t\) is the change in temperature. The symbol c stands for the specific heat. We now introduce two concepts useful in describing heat flow and temperature change.. Is Q Heat Capacity.
From www.slideserve.com
PPT Thermal Physics PowerPoint Presentation, free download ID6211191 Is Q Heat Capacity The heat capacity of a defined object is usually expressed in joules or calories and temperature in kelvin or celsius. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). The symbol c stands for the specific heat. Where \(q\) is the symbol for heat transfer (“quantity of heat”), m. Is Q Heat Capacity.
From courses.lumenlearning.com
Specific Heat Boundless Physics Is Q Heat Capacity The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). The symbol c stands for the specific heat. The heat cap acity (\(c\)) of a body of matter is the quantity of heat. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity. Is Q Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Is Q Heat Capacity The heat \(q\) transferred to cause a temperature change depends on the magnitude of the temperature change, the mass of the system, and the substance and phase. We now introduce two concepts useful in describing heat flow and temperature change. The heat cap acity (\(c\)) of a body of matter is the quantity of heat. The symbol c stands for. Is Q Heat Capacity.
From www.linstitute.net
Edexcel IGCSE Physics 复习笔记 5.2.4 Specific Heat Capacity翰林国际教育 Is Q Heat Capacity The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta t\) is the change in temperature. The heat cap acity (\(c\)) of a body of matter is the quantity of. Is Q Heat Capacity.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube Is Q Heat Capacity Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta t\) is the change in temperature. We now introduce two concepts useful in describing heat flow and temperature change. Where q is the energy added and δt is the change in temperature. The heat capacity of a defined object is. Is Q Heat Capacity.
From www.tec-science.com
Specific heat capacity of selected substances tecscience Is Q Heat Capacity The heat \(q\) transferred to cause a temperature change depends on the magnitude of the temperature change, the mass of the system, and the substance and phase. We now introduce two concepts useful in describing heat flow and temperature change. Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta. Is Q Heat Capacity.
From studylib.net
Heat Equation Is Q Heat Capacity We now introduce two concepts useful in describing heat flow and temperature change. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have. The heat capacity of a defined object is usually expressed in joules or calories and temperature in kelvin or celsius. The. Is Q Heat Capacity.
From studylib.net
Heat Capacity and Specific Heat Worksheet 1 3/3/04 12641 PM Is Q Heat Capacity Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta t\) is the change in temperature. The heat capacity of a defined object is usually expressed in joules or calories and temperature in kelvin or celsius. The specific heat capacity is intensive, and does not depend on the quantity, but. Is Q Heat Capacity.
From haipernews.com
How To Calculate Specific Heat Capacity Without Q Haiper Is Q Heat Capacity The symbol c stands for the specific heat. Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta t\) is the change in temperature. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Where q is the energy added. Is Q Heat Capacity.
From www.slideserve.com
PPT Specific Heat PowerPoint Presentation, free download ID3721637 Is Q Heat Capacity The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have. The heat \(q\) transferred to cause a temperature change depends on the magnitude of the temperature change, the mass of the system, and the substance and phase. The heat capacity of a defined object. Is Q Heat Capacity.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Is Q Heat Capacity We now introduce two concepts useful in describing heat flow and temperature change. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have. Where q is the energy added and δt is the change in temperature. The heat capacity of a defined object is. Is Q Heat Capacity.
From www.tes.com
Specific heat capacity complete lesson (GCSE 19) Teaching Resources Is Q Heat Capacity The symbol c stands for the specific heat. Where q is the energy added and δt is the change in temperature. The heat \(q\) transferred to cause a temperature change depends on the magnitude of the temperature change, the mass of the system, and the substance and phase. We now introduce two concepts useful in describing heat flow and temperature. Is Q Heat Capacity.
From testbook.com
Specific Heat Capacity Learning Notes for IIT JEE Testbook Is Q Heat Capacity The heat capacity of a defined object is usually expressed in joules or calories and temperature in kelvin or celsius. Where q is the energy added and δt is the change in temperature. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have. The. Is Q Heat Capacity.
From www.slideserve.com
PPT ENERGY PowerPoint Presentation, free download ID3232966 Is Q Heat Capacity Where q is the energy added and δt is the change in temperature. The heat \(q\) transferred to cause a temperature change depends on the magnitude of the temperature change, the mass of the system, and the substance and phase. The symbol c stands for the specific heat. The formula for specific heat capacity, c, of a substance with mass. Is Q Heat Capacity.
From www.grc.nasa.gov
Specific Heats Is Q Heat Capacity We now introduce two concepts useful in describing heat flow and temperature change. The heat capacity of a defined object is usually expressed in joules or calories and temperature in kelvin or celsius. The heat cap acity (\(c\)) of a body of matter is the quantity of heat. The symbol c stands for the specific heat. Where q is the. Is Q Heat Capacity.
From ar.inspiredpencil.com
Common Heat Capacities Is Q Heat Capacity The heat \(q\) transferred to cause a temperature change depends on the magnitude of the temperature change, the mass of the system, and the substance and phase. The symbol c stands for the specific heat. The heat capacity of a defined object is usually expressed in joules or calories and temperature in kelvin or celsius. The formula for specific heat. Is Q Heat Capacity.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Is Q Heat Capacity The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Where q is the energy added and δt is the change in temperature. We now introduce two concepts useful in describing heat flow and temperature change. The specific heat capacity is intensive, and does not depend on the quantity, but. Is Q Heat Capacity.
From slidetodoc.com
SPECIFIC HEAT Calculations SPECIFIC HEAT CAPACITY The amount Is Q Heat Capacity The heat \(q\) transferred to cause a temperature change depends on the magnitude of the temperature change, the mass of the system, and the substance and phase. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). The heat cap acity (\(c\)) of a body of matter is the quantity. Is Q Heat Capacity.
From studylib.net
Worksheet Is Q Heat Capacity The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). The heat capacity of a defined object is usually expressed in joules or calories and temperature in kelvin or celsius. Where q is the energy added and δt is the change in temperature. The symbol c stands for the specific. Is Q Heat Capacity.
From www.tessshebaylo.com
Specific Heat Equation Calculator Tessshebaylo Is Q Heat Capacity The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have. The symbol c stands for the specific heat. The heat capacity of a defined. Is Q Heat Capacity.
From rosemaryqocallahan.blogspot.com
How to Calculate Specific Heat Capacity RosemaryqoCallahan Is Q Heat Capacity Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta t\) is the change in temperature. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have. We now introduce two concepts useful in describing. Is Q Heat Capacity.
From brainly.in
Distinguish between heat capacity and specific heat of a substance Is Q Heat Capacity The heat cap acity (\(c\)) of a body of matter is the quantity of heat. The heat \(q\) transferred to cause a temperature change depends on the magnitude of the temperature change, the mass of the system, and the substance and phase. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is. Is Q Heat Capacity.
From www.pearson.com
How to calculation specific heat, heat, mass, or temperature chan Is Q Heat Capacity The heat cap acity (\(c\)) of a body of matter is the quantity of heat. The heat \(q\) transferred to cause a temperature change depends on the magnitude of the temperature change, the mass of the system, and the substance and phase. We now introduce two concepts useful in describing heat flow and temperature change. The specific heat capacity is. Is Q Heat Capacity.
From byjus.com
The SI unit of specific heat capacity of a substance is Is Q Heat Capacity Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta t\) is the change in temperature. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have. Where q is the energy added and δt. Is Q Heat Capacity.
From www.slideserve.com
PPT Heat (q) PowerPoint Presentation, free download ID1551407 Is Q Heat Capacity The heat capacity of a defined object is usually expressed in joules or calories and temperature in kelvin or celsius. We now introduce two concepts useful in describing heat flow and temperature change. The heat \(q\) transferred to cause a temperature change depends on the magnitude of the temperature change, the mass of the system, and the substance and phase.. Is Q Heat Capacity.
From www.slideserve.com
PPT Chapter 17 The first law of thermodynamics PowerPoint Is Q Heat Capacity The symbol c stands for the specific heat. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta t\) is the change in temperature. The heat capacity of a defined. Is Q Heat Capacity.
From proper-cooking.info
Specific Heat Equation Is Q Heat Capacity The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Where q is the energy added and δt is the change in temperature. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have. The. Is Q Heat Capacity.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Is Q Heat Capacity Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta t\) is the change in temperature. The symbol c stands for the specific heat. Where q is the energy added and δt is the change in temperature. The heat \(q\) transferred to cause a temperature change depends on the magnitude. Is Q Heat Capacity.
From studylib.net
Specific Heat Capacity (c) Formula q = mc)T Exercises (Be careful Is Q Heat Capacity The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Where q is the energy added and δt is the change in temperature. Where \(q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\delta t\) is the change in temperature. The. Is Q Heat Capacity.