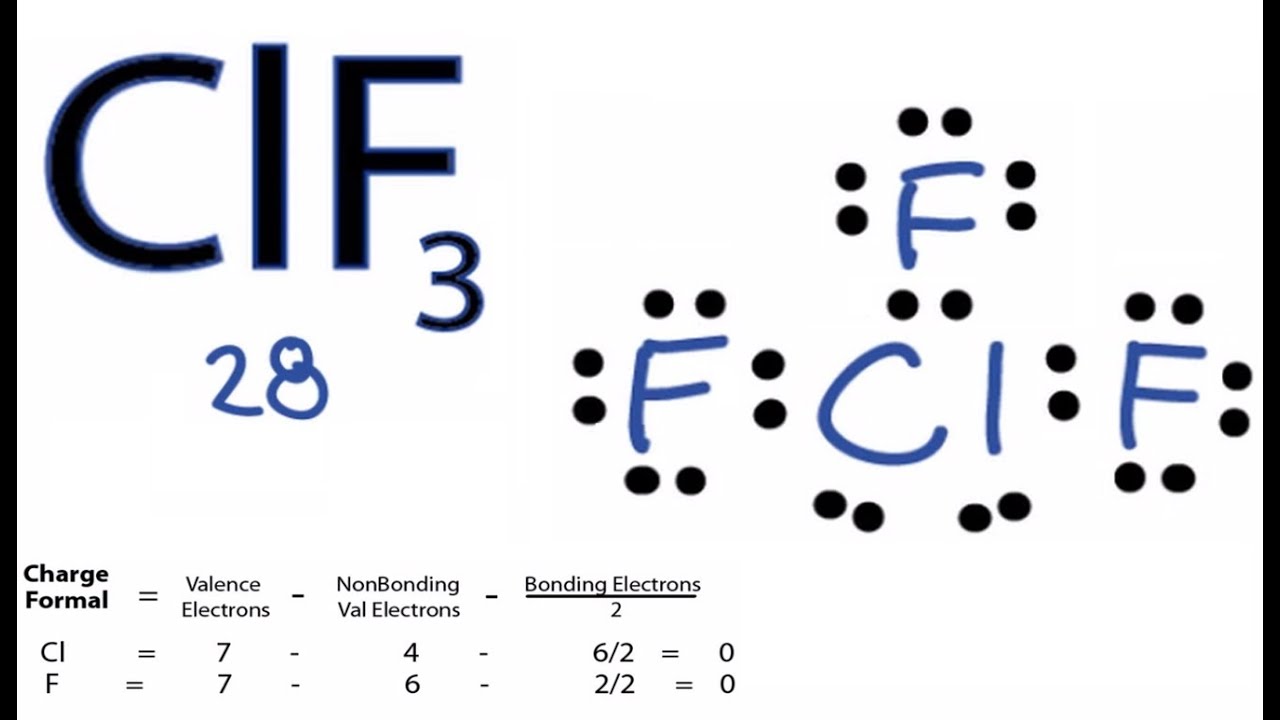
Chlorine Trifluoride Electrons . The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Chlorine’s electronic configuration is given by [ne]3s 2 3p 5. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. In addition, there are two lone pairs of electrons on the chlorine atom. One of the trigonal positions is occupied by the pair deriving. Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine and fluorine are nasty enough by themselves, but at least in. Therefore, the chlorine atom contributes 7 x 1 = 7 valence electrons.
from www.youtube.com
Therefore, the chlorine atom contributes 7 x 1 = 7 valence electrons. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. Chlorine’s electronic configuration is given by [ne]3s 2 3p 5. In addition, there are two lone pairs of electrons on the chlorine atom. Chlorine and fluorine are nasty enough by themselves, but at least in. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. One of the trigonal positions is occupied by the pair deriving.
ClF3 Lewis Structure How to Draw the Lewis Structure for ClF3 YouTube
Chlorine Trifluoride Electrons One of the trigonal positions is occupied by the pair deriving. Chlorine and fluorine are nasty enough by themselves, but at least in. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Therefore, the chlorine atom contributes 7 x 1 = 7 valence electrons. Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. One of the trigonal positions is occupied by the pair deriving. In addition, there are two lone pairs of electrons on the chlorine atom. Chlorine’s electronic configuration is given by [ne]3s 2 3p 5. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known.
From www.bigstockphoto.com
Chlorine Trifluoride Image & Photo (Free Trial) Bigstock Chlorine Trifluoride Electrons Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. Chlorine’s electronic configuration is given by [ne]3s 2 3p. Chlorine Trifluoride Electrons.
From www.numerade.com
SOLVED 5) Chlorine trifluoride has two sets of lone pairs of electrons Chlorine Trifluoride Electrons In addition, there are two lone pairs of electrons on the chlorine atom. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Chlorine’s electronic configuration is given by [ne]3s 2 3p 5. Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. One of the trigonal positions is occupied by. Chlorine Trifluoride Electrons.
From www.slideserve.com
PPT VSEPR. PowerPoint Presentation ID214953 Chlorine Trifluoride Electrons A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. In addition, there are two lone pairs of electrons on the chlorine atom. Therefore, the chlorine atom contributes 7 x 1. Chlorine Trifluoride Electrons.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Trifluoride Electrons Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. One of the trigonal positions is occupied by the pair deriving. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Chlorine and fluorine are nasty enough by themselves, but at least in. A chlorine atom has an electronegativity. Chlorine Trifluoride Electrons.
From www.numerade.com
SOLVED 5) Chlorine trifluoride has two sets of lone pairs of electrons Chlorine Trifluoride Electrons Chlorine’s electronic configuration is given by [ne]3s 2 3p 5. One of the trigonal positions is occupied by the pair deriving. In addition, there are two lone pairs of electrons on the chlorine atom. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Chlorine trifluoride (which i will subsequently refer. Chlorine Trifluoride Electrons.
From www.alamy.com
Chlorine trifluoride hires stock photography and images Alamy Chlorine Trifluoride Electrons Chlorine and fluorine are nasty enough by themselves, but at least in. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine’s electronic configuration is given by [ne]3s. Chlorine Trifluoride Electrons.
From www.youtube.com
ClF3 Hybridization (Chlorine Trifluoride) YouTube Chlorine Trifluoride Electrons Chlorine and fluorine are nasty enough by themselves, but at least in. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. One of the trigonal positions is occupied by the pair deriving. Chlorine trifluoride (which i will subsequently refer to. Chlorine Trifluoride Electrons.
From www.youtube.com
ClF3 (Chlorine trifluoride) Molecular Geometry, Bond Angles,& Electron Chlorine Trifluoride Electrons Chlorine’s electronic configuration is given by [ne]3s 2 3p 5. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. In addition, there are two lone pairs of electrons on the chlorine atom. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded. Chlorine Trifluoride Electrons.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3 Chlorine Trifluoride Electrons Therefore, the chlorine atom contributes 7 x 1 = 7 valence electrons. Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. One of the trigonal positions is occupied by the pair deriving. A chlorine atom has an electronegativity of. Chlorine Trifluoride Electrons.
From www.youtube.com
ClF3 Lewis Structure How to Draw the Lewis Structure for ClF3 YouTube Chlorine Trifluoride Electrons Therefore, the chlorine atom contributes 7 x 1 = 7 valence electrons. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. In addition, there are two lone pairs of electrons on the chlorine atom. Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. Chlorine and fluorine are nasty. Chlorine Trifluoride Electrons.
From www.chegg.com
Solved 5. Molecule CIF, (chlorine trifluoride is an Chlorine Trifluoride Electrons Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine’s electronic configuration is given by [ne]3s 2 3p 5. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. In addition, there are two lone pairs of electrons. Chlorine Trifluoride Electrons.
From www.youtube.com
ClF3 Lewis Structure (Chlorine Trifluoride) YouTube Chlorine Trifluoride Electrons Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. Chlorine and fluorine are nasty enough by themselves, but at least in. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. One of the trigonal positions is occupied by the pair deriving. Chlorine’s electronic configuration is given by. Chlorine Trifluoride Electrons.
From www.studocu.com
Chlorine Trifluoride M.O. Diagram CHEM12A Studocu Chlorine Trifluoride Electrons One of the trigonal positions is occupied by the pair deriving. Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine and fluorine are nasty enough by themselves, but at least in. Therefore, the chlorine atom contributes 7 x. Chlorine Trifluoride Electrons.
From favpng.com
Chlorine Trifluoride Dichlorine Monoxide Chemistry Chloride, PNG Chlorine Trifluoride Electrons A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. One of the trigonal positions is occupied by the pair deriving. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Therefore, the chlorine atom contributes 7 x 1 = 7. Chlorine Trifluoride Electrons.
From byjus.com
Hybridization of ClF3 Hybridization of Cl in Chlorine Trifluoride Chlorine Trifluoride Electrons One of the trigonal positions is occupied by the pair deriving. In addition, there are two lone pairs of electrons on the chlorine atom. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Therefore, the chlorine atom contributes 7 x 1 = 7 valence electrons. Chlorine trifluoride comprises three fluorine. Chlorine Trifluoride Electrons.
From www.dreamstime.com
Chlorine Trifluoride Gas Molecule Icon Stock Illustration Chlorine Trifluoride Electrons Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Therefore, the chlorine atom contributes 7 x 1 = 7 valence electrons. Chlorine and fluorine are nasty enough by themselves, but at least in. One of the trigonal positions is occupied. Chlorine Trifluoride Electrons.
From www.shutterstock.com
Chlorine Trifluoride Molecular Structure Formula Periodic Stock Vector Chlorine Trifluoride Electrons In addition, there are two lone pairs of electrons on the chlorine atom. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. One of the trigonal positions is occupied by the pair deriving. Chlorine’s electronic configuration is. Chlorine Trifluoride Electrons.
From mungfali.com
Chlorine Orbital Diagram Chlorine Trifluoride Electrons One of the trigonal positions is occupied by the pair deriving. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine’s electronic configuration is given by [ne]3s 2 3p 5. Therefore, the chlorine atom contributes 7 x 1 = 7 valence electrons. In addition, there are two lone pairs of electrons on the chlorine atom. Chlorine trifluoride. Chlorine Trifluoride Electrons.
From de-academic.com
Chlor(III)fluorid Chlorine Trifluoride Electrons The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Chlorine’s electronic configuration is given by [ne]3s 2 3p 5. In addition, there are two lone pairs of electrons on the chlorine atom. Therefore, the chlorine atom contributes 7 x 1 = 7 valence electrons. Chlorine trifluoride (which i. Chlorine Trifluoride Electrons.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3 Chlorine Trifluoride Electrons The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. One of the trigonal positions is occupied by the pair deriving. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. In addition, there are two lone. Chlorine Trifluoride Electrons.
From www.youtube.com
Is ClF3 Polar or Nonpolar (Chlorine trifluoride) YouTube Chlorine Trifluoride Electrons Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. One of the trigonal positions is occupied by the pair deriving. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. In addition, there are two lone. Chlorine Trifluoride Electrons.
From ck12.org
Molecular Geometry CK12 Foundation Chlorine Trifluoride Electrons One of the trigonal positions is occupied by the pair deriving. Chlorine’s electronic configuration is given by [ne]3s 2 3p 5. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride comprises three fluorine atoms. Chlorine Trifluoride Electrons.
From www.numerade.com
SOLVED Determine the number of valence electrons in ClF₃, chlorine Chlorine Trifluoride Electrons Chlorine’s electronic configuration is given by [ne]3s 2 3p 5. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. In addition, there are two lone pairs of electrons on the chlorine atom. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising. Chlorine Trifluoride Electrons.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Trifluoride Electrons Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. One of the trigonal positions is occupied by the pair. Chlorine Trifluoride Electrons.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Trifluoride Electrons The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. One of the trigonal positions is occupied by the pair deriving. In addition, there are two lone pairs of electrons on the chlorine atom. Chlorine’s electronic configuration is given by [ne]3s 2 3p 5. Chlorine trifluoride (which i will. Chlorine Trifluoride Electrons.
From interestingengineering.com
Chlorine Trifluoride The Compound That Can Even Set Fire to Glass Chlorine Trifluoride Electrons In addition, there are two lone pairs of electrons on the chlorine atom. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride (which i. Chlorine Trifluoride Electrons.
From chemicalportal.ru
Фторид хлора (III) — свойства, получение и применение Chlorine Trifluoride Electrons The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Therefore, the chlorine atom contributes 7 x 1 = 7 valence electrons. Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. Chlorine and fluorine are nasty enough by themselves, but at least in. Chlorine trifluoride (which i will. Chlorine Trifluoride Electrons.
From www.studocu.com
Chemistry 101 Chapter 7 Part 3 Draw the Lewis structure of ClF₃ Chlorine Trifluoride Electrons One of the trigonal positions is occupied by the pair deriving. Chlorine’s electronic configuration is given by [ne]3s 2 3p 5. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. Therefore, the chlorine atom contributes 7 x 1 = 7 valence electrons. Chlorine trifluoride comprises three fluorine atoms and one. Chlorine Trifluoride Electrons.
From bilag.xxl.no
Draw The Lewis Structure For The Chlorine Trifluoride Molecule Chlorine Trifluoride Electrons Therefore, the chlorine atom contributes 7 x 1 = 7 valence electrons. Chlorine’s electronic configuration is given by [ne]3s 2 3p 5. Chlorine and fluorine are nasty enough by themselves, but at least in. One of the trigonal positions is occupied by the pair deriving. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the. Chlorine Trifluoride Electrons.
From www.bigstockphoto.com
Chlorine Trifluoride Image & Photo (Free Trial) Bigstock Chlorine Trifluoride Electrons Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. Chlorine and fluorine are nasty enough by themselves, but at least in. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. In addition, there are two lone pairs of electrons on the chlorine atom. Therefore, the chlorine atom contributes 7. Chlorine Trifluoride Electrons.
From brokeasshome.com
Periodic Table Chlorine Electrons Chlorine Trifluoride Electrons The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. Chlorine’s electronic configuration is given by [ne]3s 2 3p 5. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds. Chlorine Trifluoride Electrons.
From favpng.com
Lewis Structure Chlorine Valence Electron Diagram, PNG, 2000x2000px Chlorine Trifluoride Electrons Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. One of the trigonal positions is occupied by the pair deriving. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine’s electronic configuration is given by [ne]3s 2 3p 5. Chlorine and fluorine are nasty enough by themselves, but. Chlorine Trifluoride Electrons.
From www.alamy.com
Chlorine gas white background hires stock photography and images Alamy Chlorine Trifluoride Electrons In addition, there are two lone pairs of electrons on the chlorine atom. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative has a value of. One of the trigonal positions is occupied by the pair deriving. Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. The lewis structure of clf3, or chlorine. Chlorine Trifluoride Electrons.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Chlorine Trifluoride Electrons In addition, there are two lone pairs of electrons on the chlorine atom. Chlorine trifluoride (which i will subsequently refer to as clf 3) is one of the most reactive oxidising compounds known. Chlorine and fluorine are nasty enough by themselves, but at least in. A chlorine atom has an electronegativity of 3.16 whereas a fluorine atom, being highly electronegative. Chlorine Trifluoride Electrons.
From www.youtube.com
ClO Lewis Structure How to Draw the Lewis Structure for ClO YouTube Chlorine Trifluoride Electrons The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Therefore, the chlorine atom contributes 7 x 1 = 7 valence electrons. Chlorine trifluoride comprises three fluorine atoms and one chlorine atom. In addition, there are two lone pairs of electrons on the chlorine atom. One of the trigonal. Chlorine Trifluoride Electrons.