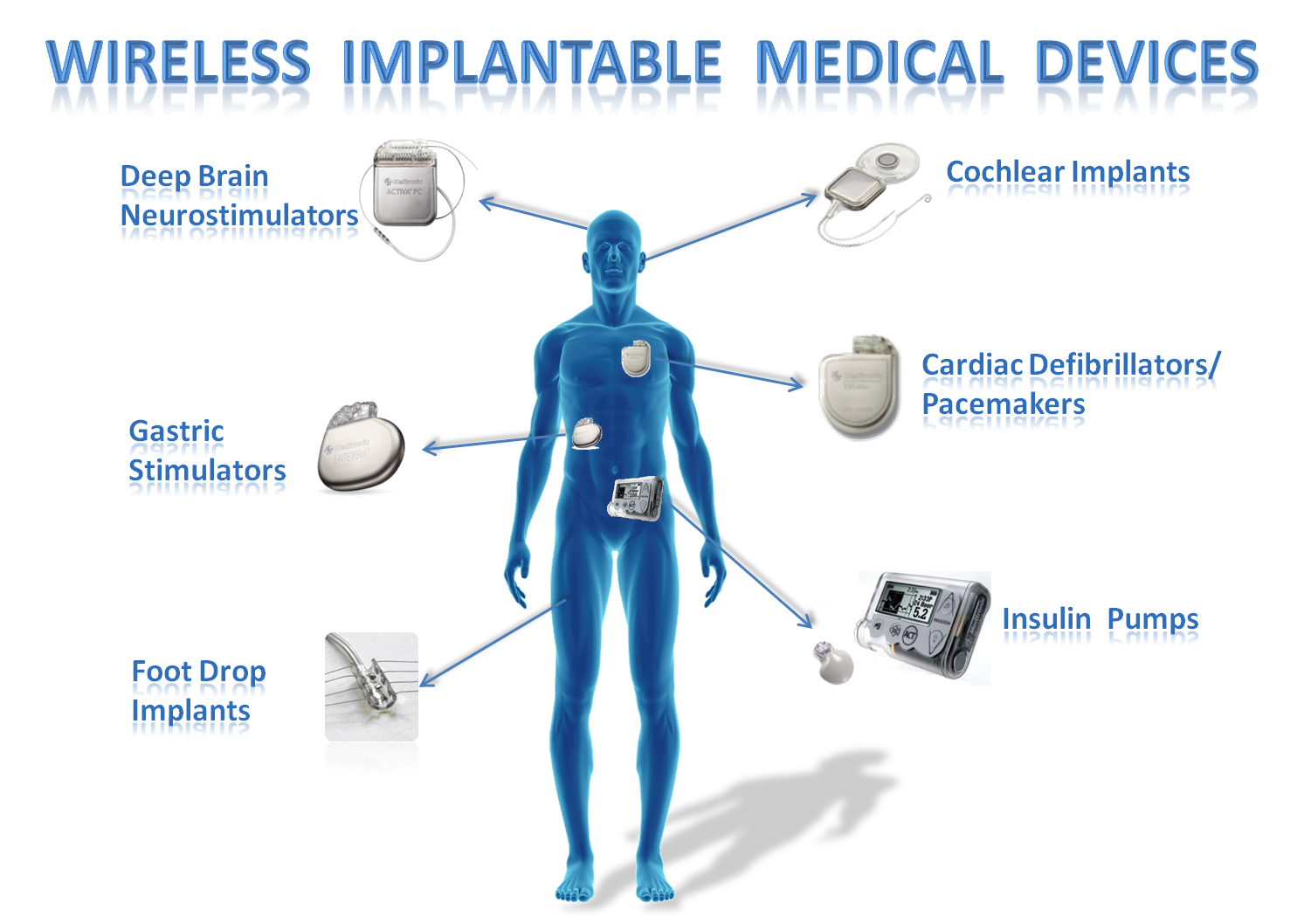
Medical Device Implant . When the medical device is implanted in the human body, the surrounding environment immediately responds to the. The medical devices regulation applies since 26 may 2021. Class iii is the most scientifically rigorous classification of medical devices and encompasses most of the orthopedic implants. Present guidelines for device implantation: Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy This article outlines the regulatory requirements for. Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. Manufacturers must comply with the regulation when placing new medical devices. As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants.
from groups.csail.mit.edu
Manufacturers must comply with the regulation when placing new medical devices. As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants. Class iii is the most scientifically rigorous classification of medical devices and encompasses most of the orthopedic implants. When the medical device is implanted in the human body, the surrounding environment immediately responds to the. Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. The medical devices regulation applies since 26 may 2021. This article outlines the regulatory requirements for. Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy Present guidelines for device implantation:
IMD Shield Securing Implantable Medical Devices
Medical Device Implant Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. Manufacturers must comply with the regulation when placing new medical devices. Class iii is the most scientifically rigorous classification of medical devices and encompasses most of the orthopedic implants. This article outlines the regulatory requirements for. Present guidelines for device implantation: The medical devices regulation applies since 26 may 2021. Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. When the medical device is implanted in the human body, the surrounding environment immediately responds to the. Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants.
From www.wevolver.com
Custom 3D Printed Medical Devices Trends and Opportunities Medical Device Implant Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. When the medical device is implanted in the human body, the surrounding environment immediately responds to the. As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants. Present guidelines for. Medical Device Implant.
From www.finetubes.co.uk
ImplantGrade Tubes for Medical Devices Medical Device Implant As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants. The medical devices regulation applies since 26 may 2021. This article outlines the regulatory requirements for. Manufacturers must comply with the regulation when placing new medical devices. Class iii is the most scientifically rigorous classification of medical devices and encompasses most. Medical Device Implant.
From www.researchgate.net
Implantable medical devices. (a) an artificial pacemaker implanted in a Medical Device Implant Class iii is the most scientifically rigorous classification of medical devices and encompasses most of the orthopedic implants. This article outlines the regulatory requirements for. The medical devices regulation applies since 26 may 2021. Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. When the medical device is. Medical Device Implant.
From www.indiamart.com
RPC Stainless Steel Medical Implant, For orthopedic, Rs 10 /piece ID Medical Device Implant This article outlines the regulatory requirements for. When the medical device is implanted in the human body, the surrounding environment immediately responds to the. Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. Class iii is the most scientifically rigorous classification of medical devices and encompasses most of. Medical Device Implant.
From lubrizolcdmo.com
LongActing Drug Delivery Injections, Implants, and Combination Medical Device Implant This article outlines the regulatory requirements for. Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants. Class iii is the most scientifically rigorous classification of medical devices and encompasses most of the orthopedic implants. Manufacturers must. Medical Device Implant.
From medicalxpress.com
A novel active photonic wireless system to power medical implants Medical Device Implant Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy Class iii is the most scientifically rigorous classification of medical devices and encompasses most of the orthopedic implants. When the medical device is implanted in the human body, the surrounding environment immediately responds to the. Manufacturers must comply with the regulation when placing new medical devices.. Medical Device Implant.
From www.mdi-llc.net
Implants Medical & Orthopedic Implants Medical Device & Implants Medical Device Implant Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy This article outlines the regulatory requirements for. Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. Present guidelines for device implantation: Manufacturers must comply with the regulation when placing new medical devices. When the. Medical Device Implant.
From cacvi.org
Pacemakers & Defibrillators Center for Advanced Cardiac and Vascular Medical Device Implant Present guidelines for device implantation: Class iii is the most scientifically rigorous classification of medical devices and encompasses most of the orthopedic implants. As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants. Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy When the medical device. Medical Device Implant.
From www.aranbiomedical.com
HighPrecision Elastomeric Coating for Implantable Medical Devices Medical Device Implant When the medical device is implanted in the human body, the surrounding environment immediately responds to the. As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants. Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy Class iii is the most scientifically rigorous classification of medical. Medical Device Implant.
From www.mdi-llc.net
Implants Medical & Orthopedic Implants Medical Device & Implants Medical Device Implant When the medical device is implanted in the human body, the surrounding environment immediately responds to the. As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants. Manufacturers must comply with the regulation when placing new medical devices. Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization. Medical Device Implant.
From www.latimes.com
Photos Southland surgeons implant Watchman heart device after it gets Medical Device Implant Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy Manufacturers must comply with the regulation when placing new medical devices. The medical devices regulation applies since 26 may 2021. Class iii is the most scientifically rigorous classification of medical devices and encompasses most of the orthopedic implants. When the medical device is implanted in the. Medical Device Implant.
From www.youtube.com
WATCHMAN Implantation Technique YouTube Medical Device Implant As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants. The medical devices regulation applies since 26 may 2021. Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. This article outlines the regulatory requirements for. Clinical considerations and clinical. Medical Device Implant.
From medium.com
Dental Implant The Methods and The Further Advancements by Captivate Medical Device Implant Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy The medical devices regulation applies since 26 may 2021. When the medical device is implanted in the human body, the surrounding environment immediately responds to the. As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants. Present. Medical Device Implant.
From www.xilloc.com
Xilloc Medical B.V. Cranial Implant Patient Specific Implant Titanium Medical Device Implant This article outlines the regulatory requirements for. As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants. Manufacturers must comply with the regulation when placing new medical devices. Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy When the medical device is implanted in the human. Medical Device Implant.
From gdfeipin.com
implantablemedicaldevicesmedical GdfeiPin Medical Device Implant Manufacturers must comply with the regulation when placing new medical devices. Class iii is the most scientifically rigorous classification of medical devices and encompasses most of the orthopedic implants. Present guidelines for device implantation: The medical devices regulation applies since 26 may 2021. Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy When the medical. Medical Device Implant.
From www.nsmedicaldevices.com
Implant dentistry launches Nobel Biocare Xeal and TiUltra surfaces in Medical Device Implant When the medical device is implanted in the human body, the surrounding environment immediately responds to the. As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants. The medical devices regulation applies since 26 may 2021. Class iii is the most scientifically rigorous classification of medical devices and encompasses most of. Medical Device Implant.
From www.mdi-llc.net
Implants Medical & Orthopedic Implants Medical Device & Implants Medical Device Implant Present guidelines for device implantation: This article outlines the regulatory requirements for. Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants. Clinical considerations and clinical challenges from pacing, implantable. Medical Device Implant.
From www.medicaldevice-network.com
New implant uses microcurrent to strengthen heart muscle Medical Device Implant Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. This article outlines the regulatory requirements for. When the medical device is implanted in the human body, the surrounding environment immediately responds to the. As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2]. Medical Device Implant.
From ar.inspiredpencil.com
Medical Implants Medical Device Implant The medical devices regulation applies since 26 may 2021. Class iii is the most scientifically rigorous classification of medical devices and encompasses most of the orthopedic implants. This article outlines the regulatory requirements for. Present guidelines for device implantation: As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants. Manufacturers must. Medical Device Implant.
From www.mdi-llc.net
Implants Medical & Orthopedic Implants Medical Device & Implants Medical Device Implant Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. This article outlines the regulatory requirements for. Class iii is the most scientifically rigorous classification of medical devices and encompasses most of the orthopedic implants. Present guidelines for device implantation: As of may 26, 2021, the new european medical. Medical Device Implant.
From www.mdpi.com
Energies Free FullText Energy Harvesting in Implantable and Medical Device Implant Class iii is the most scientifically rigorous classification of medical devices and encompasses most of the orthopedic implants. Present guidelines for device implantation: This article outlines the regulatory requirements for. Manufacturers must comply with the regulation when placing new medical devices. As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants.. Medical Device Implant.
From www.watchman.com
The Implant Procedure WATCHMAN Device Medical Device Implant Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. Present guidelines for device implantation: As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants. Class iii is the most scientifically rigorous classification of medical devices and encompasses most of. Medical Device Implant.
From www.ndcs.com.sg
Next generation cardiac implantable electronic devices SingHealth Medical Device Implant The medical devices regulation applies since 26 may 2021. Manufacturers must comply with the regulation when placing new medical devices. Present guidelines for device implantation: Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy When the medical device is implanted in the human body, the surrounding environment immediately responds to the. Learn about implantable medical. Medical Device Implant.
From www.mdi-llc.net
Implants Medical & Orthopedic Implants Medical Device & Implants Medical Device Implant The medical devices regulation applies since 26 may 2021. When the medical device is implanted in the human body, the surrounding environment immediately responds to the. Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy This article outlines the regulatory requirements for. Class iii is the most scientifically rigorous classification of medical devices and encompasses. Medical Device Implant.
From www.uwhealth.org
Watchman implant Treatments UW Health Medical Device Implant The medical devices regulation applies since 26 may 2021. Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy Manufacturers must comply with the regulation when placing new medical devices. This article outlines the regulatory. Medical Device Implant.
From www.healthcarepackaging.com
Implantable Medical Devices Market to show Solid Global Growth Medical Device Implant Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy This article outlines the regulatory requirements for. Class iii is the most scientifically rigorous classification of medical devices and encompasses most of the orthopedic implants. Manufacturers must comply with the regulation when placing new medical devices. As of may 26, 2021, the new european medical device. Medical Device Implant.
From www.mdi-llc.net
Implants Medical & Orthopedic Implants Medical Device & Implants Medical Device Implant As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants. This article outlines the regulatory requirements for. Manufacturers must comply with the regulation when placing new medical devices. Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy The medical devices regulation applies since 26 may 2021.. Medical Device Implant.
From www.mergemed.com
Implants Merege Medical Device Studio Medical Device Implant Manufacturers must comply with the regulation when placing new medical devices. Class iii is the most scientifically rigorous classification of medical devices and encompasses most of the orthopedic implants. Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator,. Medical Device Implant.
From news.mit.edu
Design prevents buildup of scar tissue around medical implants MIT Medical Device Implant The medical devices regulation applies since 26 may 2021. Class iii is the most scientifically rigorous classification of medical devices and encompasses most of the orthopedic implants. This article outlines the regulatory requirements for. Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. Clinical considerations and clinical challenges. Medical Device Implant.
From www.machinedesign.com
FDA Approves Implant for Adults with AbovetheKnee Amputations Medical Device Implant Manufacturers must comply with the regulation when placing new medical devices. The medical devices regulation applies since 26 may 2021. When the medical device is implanted in the human body, the surrounding environment immediately responds to the. Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy Class iii is the most scientifically rigorous classification of. Medical Device Implant.
From www.mdi-llc.net
WorldClass Medical Device Manufacturer Medical Device & Implants Medical Device Implant The medical devices regulation applies since 26 may 2021. As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants. This article outlines the regulatory requirements for. When the medical device is implanted in the human body, the surrounding environment immediately responds to the. Learn about implantable medical devices for those with. Medical Device Implant.
From wysscenter.ch
Implanted devices could improve epilepsy management — Wyss Center Medical Device Implant Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. This article outlines the regulatory requirements for. Manufacturers must comply with the regulation when placing new medical devices. Class iii is the most scientifically rigorous classification of medical devices and encompasses most of the orthopedic implants. Clinical considerations and. Medical Device Implant.
From groups.csail.mit.edu
IMD Shield Securing Implantable Medical Devices Medical Device Implant Clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy Manufacturers must comply with the regulation when placing new medical devices. This article outlines the regulatory requirements for. Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. Present guidelines for device implantation: Class iii. Medical Device Implant.
From www.mdi-llc.net
Implants Medical & Orthopedic Implants Medical Device & Implants Medical Device Implant The medical devices regulation applies since 26 may 2021. Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. Present guidelines for device implantation: When the medical device is implanted in the human body, the surrounding environment immediately responds to the. Clinical considerations and clinical challenges from pacing, implantable. Medical Device Implant.
From engmedsys.net
Implants Engineered Medical Systems Medical Device Implant This article outlines the regulatory requirements for. Learn about implantable medical devices for those with heart disease, such as left ventricular assist device (lvad), pacemaker and implantable cardioverter. Manufacturers must comply with the regulation when placing new medical devices. As of may 26, 2021, the new european medical device regulation (eu)2017/7458 mdr) [1], [2] also applies to implants. When the. Medical Device Implant.