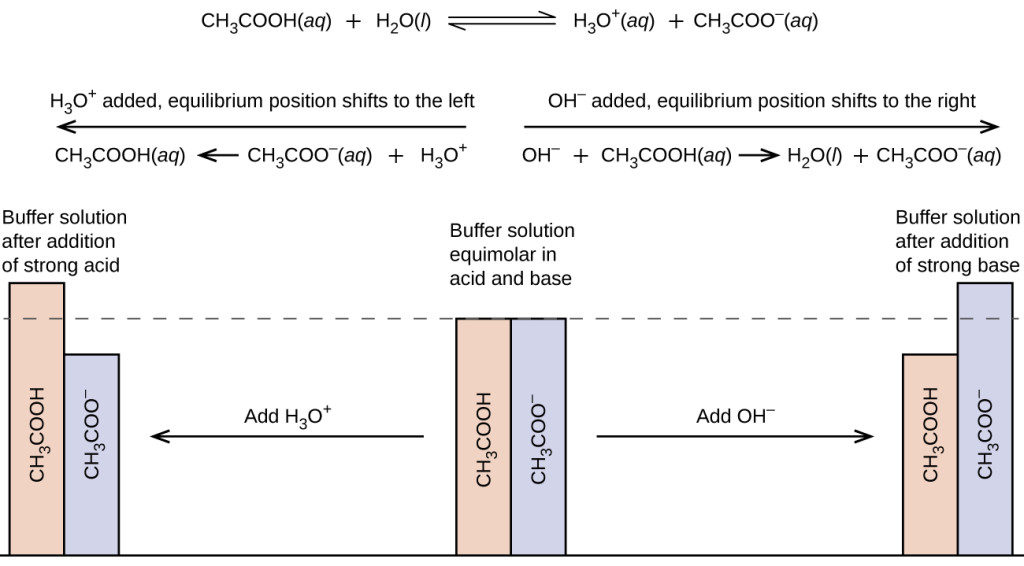
Using The Buffer . Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers allow chemists to maintain a specific ph range for a reaction. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: It is able to neutralize small amounts of added acid or base, thus. A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial.
from courses.lumenlearning.com
A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Buffers allow chemists to maintain a specific ph range for a reaction.
Buffers Chemistry for Majors
Using The Buffer It is able to neutralize small amounts of added acid or base, thus. Buffers allow chemists to maintain a specific ph range for a reaction. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small amounts of added acid or base, thus.
From digitalreachplatform.com
How To Use Buffer in 6 Minutes (A StepbyStep Guide) Using The Buffer Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A buffer solution resists a change in ph from the addition of a small amount of acid or base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.. Using The Buffer.
From www.youtube.com
14.10 Buffers Solutions that Resist pH Change YouTube Using The Buffer Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Using The Buffer.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Using The Buffer Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A buffer solution is one. Using The Buffer.
From rnbo.cycling74.com
Using Buffers Cycling '74 Using The Buffer A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution resists a change in ph from the addition of. Using The Buffer.
From www.cookedgoosecatering.com
Picking a Sandwich Buffet Menu Cooked Goose Catering Using The Buffer Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph change upon the addition of an acidic. Using The Buffer.
From ar.inspiredpencil.com
Party Buffet Using The Buffer A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers allow chemists to maintain a specific ph range. Using The Buffer.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Using The Buffer Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. Either a weak acid plus a salt derived from that weak acid, or a weak base plus. Using The Buffer.
From www.vrogue.co
Lab 8 How Do Buffers Work Lab 9 How Do Buffers Work T vrogue.co Using The Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers allow chemists to maintain a specific ph range for a reaction. A buffer solution resists a change in ph from the addition of a small amount of acid or base. This characteristic makes buffers important in biological and chemical applications. Using The Buffer.
From www.slideserve.com
PPT Chapter 17a PowerPoint Presentation, free download ID4339402 Using The Buffer A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A buffer is a solution that can resist ph. Using The Buffer.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Using The Buffer It is able to neutralize small amounts of added acid or base, thus. Buffers allow chemists to maintain a specific ph range for a reaction. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A buffer. Using The Buffer.
From courses.lumenlearning.com
Buffers Chemistry for Majors Using The Buffer A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. Buffers allow chemists to maintain a. Using The Buffer.
From studentlesson.com
How To Use A Buffer On A Car (using A Handheld Buffer) Studentlesson Using The Buffer A buffer solution resists a change in ph from the addition of a small amount of acid or base. It is able to neutralize small amounts of added acid or base, thus. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. Buffers allow chemists to maintain a. Using The Buffer.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation Using The Buffer A buffer solution resists a change in ph from the addition of a small amount of acid or base. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. It is able to neutralize small amounts of added acid or base,. Using The Buffer.
From remadeby.blogspot.com
Buffet table set up ideas Made by Self Using The Buffer A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A buffer solution is one. Using The Buffer.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Using The Buffer Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers resist dramatic changes. Using The Buffer.
From www.chegg.com
Solved Weak Acid solution used to prepare buffers a and b Using The Buffer Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers. Using The Buffer.
From scienceinfo.com
Buffer Solution Types, Properties, and Uses Using The Buffer Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. Buffers allow chemists to maintain a specific ph range for a reaction. This characteristic makes buffers important in biological and chemical applications where ph. Using The Buffer.
From pdfprof.com
buffer capacity experiment procedure Using The Buffer Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of. Using The Buffer.
From alekasgettogether.com
9 Tips for Setting Up a Buffet Table Aleka's Get Together Using The Buffer A buffer solution resists a change in ph from the addition of a small amount of acid or base. A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A mixture. Using The Buffer.
From app.jove.com
Buffers, Buffer Components and Buffer Action Chemistry JoVe Using The Buffer This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A buffer is a solution that can resist ph. Using The Buffer.
From www.youtube.com
NCEA L3 Chem Buffers, Buffer Region & Preparation of Buffer Solutions Using The Buffer This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called. Using The Buffer.
From rnbo.cycling74.com
Using Buffers Cycling '74 Using The Buffer This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A buffer solution is one in which the ph. Using The Buffer.
From reactivex.io
ReactiveX Buffer operator Using The Buffer It is able to neutralize small amounts of added acid or base, thus. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. Buffers allow chemists to maintain a specific ph range for a reaction. This characteristic makes buffers important in. Using The Buffer.
From www.youtube.com
What is Buffer ? Why Buffer and TriState Buffers are used in Digital Using The Buffer Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. Buffers allow chemists to maintain a specific ph range for a reaction. A buffer is a solution that can resist ph change upon the. Using The Buffer.
From www.youtube.com
Tristate buffer what is tristate buffer inverting non inverting Using The Buffer Buffers allow chemists to maintain a specific ph range for a reaction. A buffer solution resists a change in ph from the addition of a small amount of acid or base. It is able to neutralize small amounts of added acid or base, thus. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Either. Using The Buffer.
From chemistryteory.blogspot.com
chemistry How to Prepare Buffer Solutions? Using The Buffer Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. Buffers allow chemists to maintain a specific ph range for a reaction. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. Using The Buffer.
From www.croquelune-mariage.com
Catering Services Guidelines to Make For a Buffet Catering Make Using The Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is one in which the ph of the solution is resistant to small additions of either. Using The Buffer.
From www.slideshare.net
Buffers in chemical analysis, types of buffers Using The Buffer Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A buffer solution resists a change in ph from the addition of a small amount of acid or base. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of. Using The Buffer.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Using The Buffer Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. Buffers allow chemists to maintain a specific ph range for a reaction. This. Using The Buffer.
From vetmarketingpro.com
5 Benefits of Using Buffer Using The Buffer Either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak. A buffer solution resists a change in ph from the addition of a small amount of acid or base. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. It is able to. Using The Buffer.
From www.youtube.com
How to Make and pH Buffers YouTube Using The Buffer A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers allow chemists to maintain a specific ph range for a reaction.. Using The Buffer.
From wiringfixarrishes.z21.web.core.windows.net
Tri State Buffer Circuit Diagram Using The Buffer It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer solution resists a change in ph from the addition of a. Using The Buffer.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Using The Buffer This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Buffers allow chemists to maintain a specific ph range for a reaction. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes:. Using The Buffer.
From offbeatmyrtlebeach.blogspot.com
If you like Southern Style Comfort Foods You'll love this Buffet in Using The Buffer A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Buffers allow chemists to maintain a specific ph range for a reaction. A buffer is a solution that can resist. Using The Buffer.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Using The Buffer Buffers allow chemists to maintain a specific ph range for a reaction. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. It is able to neutralize small amounts of added acid or base, thus. Buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A buffer solution resists a change. Using The Buffer.