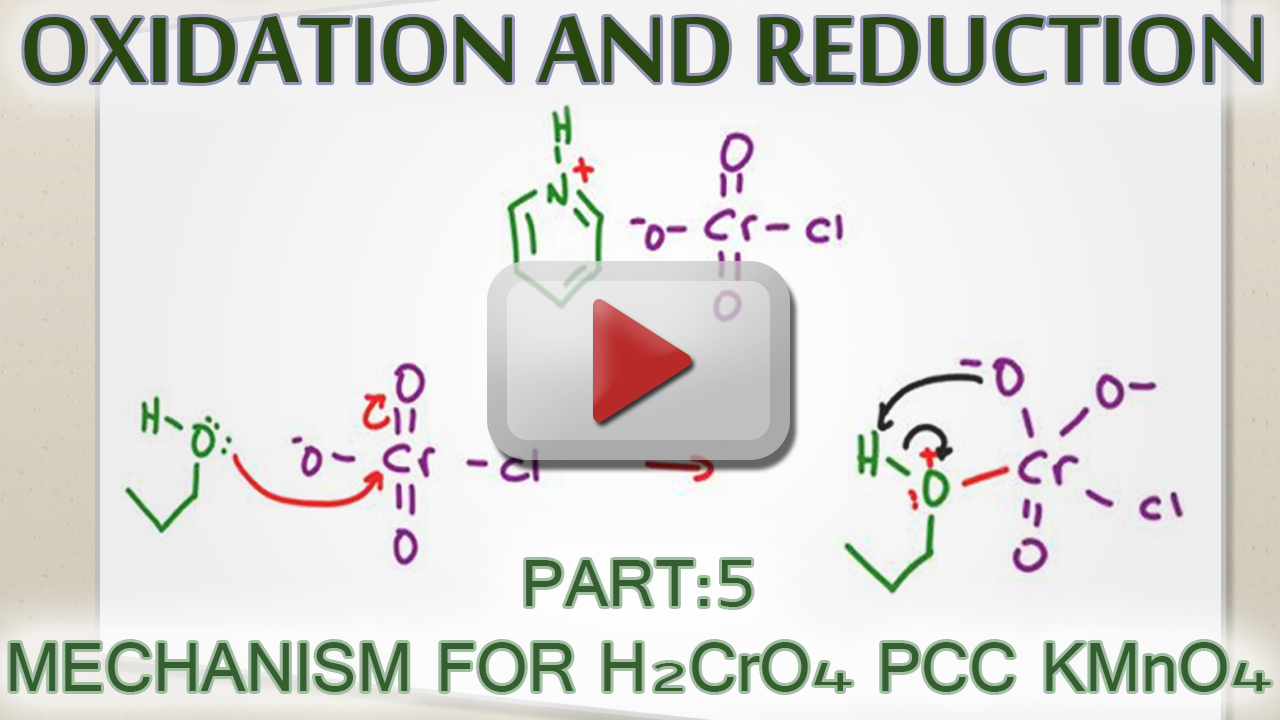
Alcohol Test With Oxidation . In these practice problems, we will discuss the oxidation of alcohols using oxidizing agents such as pcc, kmno4, na2cr2o7, swern, dmp, and their mechanisms. The rate of oxidation varies. This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. Identify the alcohol needed to prepare a given aldehyde, ketone or carboxylic acid by simple. Secondary alcohols can be oxidised to. One easy way of distinguishing between a primary and a secondary alcohol is to test for the initial products of oxidation for each alcohol: Primary alcohol molecules can be oxidised into aldehydes and carboxylic acids while secondary alcohols are oxidised to ketones. Primary alcohols can be oxidised to form aldehydes which can undergo further oxidation to form carboxylic acids; Identify the product formed from the oxidation of a given alcohol with a specified oxidizing agent. This reaction is used to make aldehydes, ketones and. In the oxidation test, the alcohols are oxidized with sodium dichromate (na 2 cr 2 o 7). This reaction is used to make aldehydes, ketones and carboxylic acids, and as a. This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution.
from leah4sci.com
This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. This reaction is used to make aldehydes, ketones and carboxylic acids, and as a. This reaction is used to make aldehydes, ketones and. Primary alcohol molecules can be oxidised into aldehydes and carboxylic acids while secondary alcohols are oxidised to ketones. Secondary alcohols can be oxidised to. In the oxidation test, the alcohols are oxidized with sodium dichromate (na 2 cr 2 o 7). The rate of oxidation varies. This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. In these practice problems, we will discuss the oxidation of alcohols using oxidizing agents such as pcc, kmno4, na2cr2o7, swern, dmp, and their mechanisms. One easy way of distinguishing between a primary and a secondary alcohol is to test for the initial products of oxidation for each alcohol:
Alcohol Oxidation Mechanism using H2CrO4, PCC and KMnO4
Alcohol Test With Oxidation This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. Identify the alcohol needed to prepare a given aldehyde, ketone or carboxylic acid by simple. The rate of oxidation varies. This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. In the oxidation test, the alcohols are oxidized with sodium dichromate (na 2 cr 2 o 7). In these practice problems, we will discuss the oxidation of alcohols using oxidizing agents such as pcc, kmno4, na2cr2o7, swern, dmp, and their mechanisms. Primary alcohol molecules can be oxidised into aldehydes and carboxylic acids while secondary alcohols are oxidised to ketones. Identify the product formed from the oxidation of a given alcohol with a specified oxidizing agent. This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. Secondary alcohols can be oxidised to. This reaction is used to make aldehydes, ketones and carboxylic acids, and as a. This reaction is used to make aldehydes, ketones and. Primary alcohols can be oxidised to form aldehydes which can undergo further oxidation to form carboxylic acids; One easy way of distinguishing between a primary and a secondary alcohol is to test for the initial products of oxidation for each alcohol:
From leah4sci.com
Alcohol Oxidation Mechanism using H2CrO4, PCC and KMnO4 Alcohol Test With Oxidation Primary alcohols can be oxidised to form aldehydes which can undergo further oxidation to form carboxylic acids; This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. One easy way of distinguishing between a primary and a secondary alcohol is to test for the initial products of oxidation for each alcohol: Identify the alcohol. Alcohol Test With Oxidation.
From www.coursehero.com
[Solved] In the chromic acid test, the alcohol undergoes an oxidation Alcohol Test With Oxidation This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. In the oxidation test, the alcohols are oxidized with sodium dichromate (na 2 cr 2 o 7). Identify the product formed from the oxidation of a given alcohol with a specified oxidizing agent. One easy way of distinguishing between a primary and a secondary. Alcohol Test With Oxidation.
From www.slideserve.com
PPT OXIDATIONS OF ALCOHOLS PowerPoint Presentation, free download Alcohol Test With Oxidation This reaction is used to make aldehydes, ketones and carboxylic acids, and as a. This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. In the oxidation test, the alcohols are oxidized with sodium dichromate (na 2 cr 2 o 7). Primary alcohol molecules can be oxidised into aldehydes and carboxylic acids while secondary. Alcohol Test With Oxidation.
From www.organicchemistrytutor.com
Oxidation of Alcohols Overview — Organic Chemistry Tutor Alcohol Test With Oxidation Identify the alcohol needed to prepare a given aldehyde, ketone or carboxylic acid by simple. Primary alcohol molecules can be oxidised into aldehydes and carboxylic acids while secondary alcohols are oxidised to ketones. In these practice problems, we will discuss the oxidation of alcohols using oxidizing agents such as pcc, kmno4, na2cr2o7, swern, dmp, and their mechanisms. Primary alcohols can. Alcohol Test With Oxidation.
From www.chemistrysteps.com
Alcohol Oxidation Mechanisms and Practice Problems Chemistry Steps Alcohol Test With Oxidation In the oxidation test, the alcohols are oxidized with sodium dichromate (na 2 cr 2 o 7). Identify the alcohol needed to prepare a given aldehyde, ketone or carboxylic acid by simple. One easy way of distinguishing between a primary and a secondary alcohol is to test for the initial products of oxidation for each alcohol: This reaction is used. Alcohol Test With Oxidation.
From www.masterorganicchemistry.com
Alcohol Oxidation "Strong" & "Weak" Oxidants Master Organic Chemistry Alcohol Test With Oxidation In the oxidation test, the alcohols are oxidized with sodium dichromate (na 2 cr 2 o 7). Primary alcohols can be oxidised to form aldehydes which can undergo further oxidation to form carboxylic acids; This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. Identify the alcohol needed to prepare a given aldehyde, ketone. Alcohol Test With Oxidation.
From www.linstitute.net
AQA A Level Chemistry复习笔记3.5.3 Oxidation of Alcohols翰林国际教育 Alcohol Test With Oxidation The rate of oxidation varies. Primary alcohols can be oxidised to form aldehydes which can undergo further oxidation to form carboxylic acids; This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. This reaction is used to make aldehydes, ketones and carboxylic acids, and as a. Secondary alcohols can be oxidised to. In the. Alcohol Test With Oxidation.
From fineartamerica.com
Alcohol Oxidation Photograph by Andrew Lambert Photography/science Alcohol Test With Oxidation This reaction is used to make aldehydes, ketones and carboxylic acids, and as a. In the oxidation test, the alcohols are oxidized with sodium dichromate (na 2 cr 2 o 7). Identify the alcohol needed to prepare a given aldehyde, ketone or carboxylic acid by simple. Identify the product formed from the oxidation of a given alcohol with a specified. Alcohol Test With Oxidation.
From www.masterorganicchemistry.com
Alcohol Oxidation "Strong" & "Weak" Oxidants Master Organic Chemistry Alcohol Test With Oxidation Identify the product formed from the oxidation of a given alcohol with a specified oxidizing agent. The rate of oxidation varies. This reaction is used to make aldehydes, ketones and carboxylic acids, and as a. One easy way of distinguishing between a primary and a secondary alcohol is to test for the initial products of oxidation for each alcohol: Primary. Alcohol Test With Oxidation.
From www.chemistrysteps.com
Alcohol Oxidation Mechanisms and Practice Problems Chemistry Steps Alcohol Test With Oxidation Secondary alcohols can be oxidised to. In these practice problems, we will discuss the oxidation of alcohols using oxidizing agents such as pcc, kmno4, na2cr2o7, swern, dmp, and their mechanisms. Identify the alcohol needed to prepare a given aldehyde, ketone or carboxylic acid by simple. This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi). Alcohol Test With Oxidation.
From www.chegg.com
Solved Chromic Acid Test Oxidation of Alcohols Alcohol Alcohol Test With Oxidation Identify the alcohol needed to prepare a given aldehyde, ketone or carboxylic acid by simple. In these practice problems, we will discuss the oxidation of alcohols using oxidizing agents such as pcc, kmno4, na2cr2o7, swern, dmp, and their mechanisms. One easy way of distinguishing between a primary and a secondary alcohol is to test for the initial products of oxidation. Alcohol Test With Oxidation.
From www.chemistrystudent.com
Oxidation of Alcohols (ALevel) ChemistryStudent Alcohol Test With Oxidation Identify the product formed from the oxidation of a given alcohol with a specified oxidizing agent. Primary alcohol molecules can be oxidised into aldehydes and carboxylic acids while secondary alcohols are oxidised to ketones. In the oxidation test, the alcohols are oxidized with sodium dichromate (na 2 cr 2 o 7). One easy way of distinguishing between a primary and. Alcohol Test With Oxidation.
From www.youtube.com
Chemical Tests for Alcohols Lucas Test & Oxidation Tests // HSC Alcohol Test With Oxidation This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. This reaction is used to make aldehydes, ketones and. The rate of oxidation varies. Identify the alcohol needed to prepare a given aldehyde, ketone or carboxylic acid by simple. Primary alcohol molecules can be oxidised into aldehydes and carboxylic acids while secondary alcohols are. Alcohol Test With Oxidation.
From www.tes.com
Oxidation of Alcohols AS Chemistry OCR Teaching Resources Alcohol Test With Oxidation This reaction is used to make aldehydes, ketones and. This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. In the oxidation test, the alcohols are oxidized with sodium dichromate (na 2 cr 2 o 7). This reaction is used to make aldehydes, ketones and carboxylic acids, and as a. In these practice problems,. Alcohol Test With Oxidation.
From www.chemistrysteps.com
Oxidation of Alcohols Mechanisms and Practice Problems Chemistry Steps Alcohol Test With Oxidation Identify the product formed from the oxidation of a given alcohol with a specified oxidizing agent. This reaction is used to make aldehydes, ketones and carboxylic acids, and as a. Primary alcohols can be oxidised to form aldehydes which can undergo further oxidation to form carboxylic acids; Primary alcohol molecules can be oxidised into aldehydes and carboxylic acids while secondary. Alcohol Test With Oxidation.
From slideplayer.com
Alkanes, alkenes, alcohols, aldehydes and ketones ppt download Alcohol Test With Oxidation In the oxidation test, the alcohols are oxidized with sodium dichromate (na 2 cr 2 o 7). Identify the product formed from the oxidation of a given alcohol with a specified oxidizing agent. Identify the alcohol needed to prepare a given aldehyde, ketone or carboxylic acid by simple. This page looks at the oxidation of alcohols using acidified sodium or. Alcohol Test With Oxidation.
From www.chemistrysteps.com
Alcohol Oxidation Mechanisms and Practice Problems Chemistry Steps Alcohol Test With Oxidation This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. This reaction is used to make aldehydes, ketones and. The rate of oxidation varies. One easy way of distinguishing between a primary and a secondary alcohol is to test for the initial products of oxidation for each alcohol: Secondary alcohols can be oxidised to.. Alcohol Test With Oxidation.
From www.youtube.com
Determination of Alcohol Content by Dichromate Oxidation followed by Alcohol Test With Oxidation The rate of oxidation varies. This reaction is used to make aldehydes, ketones and. This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. Identify the alcohol needed to prepare a given aldehyde, ketone or carboxylic acid by simple. This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi). Alcohol Test With Oxidation.
From pixels.com
Alcohol Oxidation Photograph by Andrew Lambert Photography Alcohol Test With Oxidation Identify the product formed from the oxidation of a given alcohol with a specified oxidizing agent. In these practice problems, we will discuss the oxidation of alcohols using oxidizing agents such as pcc, kmno4, na2cr2o7, swern, dmp, and their mechanisms. Primary alcohol molecules can be oxidised into aldehydes and carboxylic acids while secondary alcohols are oxidised to ketones. This page. Alcohol Test With Oxidation.
From www.youtube.com
Identification test for Alcohol ethanol and methanol chemical test Alcohol Test With Oxidation The rate of oxidation varies. Primary alcohol molecules can be oxidised into aldehydes and carboxylic acids while secondary alcohols are oxidised to ketones. This reaction is used to make aldehydes, ketones and carboxylic acids, and as a. One easy way of distinguishing between a primary and a secondary alcohol is to test for the initial products of oxidation for each. Alcohol Test With Oxidation.
From www.chemistrysteps.com
Alcohol Oxidation Mechanisms and Practice Problems Chemistry Steps Alcohol Test With Oxidation In these practice problems, we will discuss the oxidation of alcohols using oxidizing agents such as pcc, kmno4, na2cr2o7, swern, dmp, and their mechanisms. This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. Identify the product formed from the oxidation of a given alcohol with a specified oxidizing agent. This reaction is used. Alcohol Test With Oxidation.
From www.chemistrysteps.com
Alcohol Oxidation Mechanisms and Practice Problems Chemistry Steps Alcohol Test With Oxidation This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. Identify the alcohol needed to prepare a given aldehyde, ketone or carboxylic acid by simple. This reaction is used to make aldehydes, ketones and carboxylic acids, and as a. This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi). Alcohol Test With Oxidation.
From passmyexams.co.uk
Oxidation of Ethanol Easy exam revision notes for GSCE Chemistry Alcohol Test With Oxidation This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. In the oxidation test, the alcohols are oxidized with sodium dichromate (na 2 cr 2 o 7). One easy way of distinguishing between a primary and a secondary. Alcohol Test With Oxidation.
From www.researchgate.net
Alcohol oxidation conditions. a Alcohols oxidation with oxidant. b Alcohol Test With Oxidation This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. In these practice problems, we will discuss the oxidation of alcohols using oxidizing agents such as pcc, kmno4, na2cr2o7, swern, dmp, and their mechanisms. This reaction is used to make aldehydes, ketones and. This reaction is used to make aldehydes, ketones and carboxylic acids,. Alcohol Test With Oxidation.
From www.compoundchem.com
A Guide to Oxidation Reactions of Alcohols Compound Interest Alcohol Test With Oxidation Primary alcohols can be oxidised to form aldehydes which can undergo further oxidation to form carboxylic acids; Secondary alcohols can be oxidised to. Identify the product formed from the oxidation of a given alcohol with a specified oxidizing agent. Primary alcohol molecules can be oxidised into aldehydes and carboxylic acids while secondary alcohols are oxidised to ketones. One easy way. Alcohol Test With Oxidation.
From www.w3schools.blog
Identification of Primary Alcohols W3schools Alcohol Test With Oxidation Primary alcohol molecules can be oxidised into aldehydes and carboxylic acids while secondary alcohols are oxidised to ketones. Secondary alcohols can be oxidised to. One easy way of distinguishing between a primary and a secondary alcohol is to test for the initial products of oxidation for each alcohol: The rate of oxidation varies. This page looks at the oxidation of. Alcohol Test With Oxidation.
From www.chemistrysteps.com
Alcohol Oxidation Mechanisms and Practice Problems Chemistry Steps Alcohol Test With Oxidation This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. This reaction is used to make aldehydes, ketones and. In these practice problems, we will discuss the oxidation of alcohols using oxidizing agents such as pcc, kmno4, na2cr2o7, swern, dmp, and their mechanisms. Secondary alcohols can be oxidised to. Identify the alcohol needed to. Alcohol Test With Oxidation.
From www.chegg.com
Solved Chromic Acid Test Oxidation of Alcohols Alcohol Alcohol Test With Oxidation Identify the alcohol needed to prepare a given aldehyde, ketone or carboxylic acid by simple. Identify the product formed from the oxidation of a given alcohol with a specified oxidizing agent. This reaction is used to make aldehydes, ketones and carboxylic acids, and as a. Primary alcohol molecules can be oxidised into aldehydes and carboxylic acids while secondary alcohols are. Alcohol Test With Oxidation.
From www.sciencephoto.com
Alcohol test Stock Image A500/0284 Science Photo Library Alcohol Test With Oxidation One easy way of distinguishing between a primary and a secondary alcohol is to test for the initial products of oxidation for each alcohol: In the oxidation test, the alcohols are oxidized with sodium dichromate (na 2 cr 2 o 7). Secondary alcohols can be oxidised to. Identify the product formed from the oxidation of a given alcohol with a. Alcohol Test With Oxidation.
From www.chemistrysteps.com
Oxidation of Alcohols Mechanisms and Practice Problems Chemistry Steps Alcohol Test With Oxidation Primary alcohol molecules can be oxidised into aldehydes and carboxylic acids while secondary alcohols are oxidised to ketones. This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. This reaction is used to make aldehydes, ketones and. In these practice problems, we will discuss the oxidation of alcohols using oxidizing agents such as pcc,. Alcohol Test With Oxidation.
From www.chemistrystudent.com
Oxidation of Alcohols (ALevel) ChemistryStudent Alcohol Test With Oxidation This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. This reaction is used to make aldehydes, ketones and carboxylic acids, and as a. The rate of oxidation varies. This reaction is used to make aldehydes, ketones and. Primary alcohol molecules can be oxidised into aldehydes and carboxylic acids while secondary alcohols are oxidised. Alcohol Test With Oxidation.
From www.slideserve.com
PPT OXIDATIONS OF ALCOHOLS PowerPoint Presentation, free download Alcohol Test With Oxidation Primary alcohol molecules can be oxidised into aldehydes and carboxylic acids while secondary alcohols are oxidised to ketones. Identify the product formed from the oxidation of a given alcohol with a specified oxidizing agent. This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate (vi) solution. This page looks at the oxidation of alcohols using acidified. Alcohol Test With Oxidation.
From www.youtube.com
4 3 11 oxidation of alcohols dichromate YouTube Alcohol Test With Oxidation Secondary alcohols can be oxidised to. Primary alcohols can be oxidised to form aldehydes which can undergo further oxidation to form carboxylic acids; In these practice problems, we will discuss the oxidation of alcohols using oxidizing agents such as pcc, kmno4, na2cr2o7, swern, dmp, and their mechanisms. The rate of oxidation varies. This reaction is used to make aldehydes, ketones. Alcohol Test With Oxidation.
From www.youtube.com
Alcohols Advanced 5. Oxidation Test 1ary & 2ary from 3ary. YouTube Alcohol Test With Oxidation This reaction is used to make aldehydes, ketones and. Identify the alcohol needed to prepare a given aldehyde, ketone or carboxylic acid by simple. In the oxidation test, the alcohols are oxidized with sodium dichromate (na 2 cr 2 o 7). This reaction is used to make aldehydes, ketones and carboxylic acids, and as a. Primary alcohol molecules can be. Alcohol Test With Oxidation.
From fineartamerica.com
Oxidations Of Alcohols Photograph by Andrew Lambert Photography Alcohol Test With Oxidation Identify the product formed from the oxidation of a given alcohol with a specified oxidizing agent. One easy way of distinguishing between a primary and a secondary alcohol is to test for the initial products of oxidation for each alcohol: This reaction is used to make aldehydes, ketones and carboxylic acids, and as a. In the oxidation test, the alcohols. Alcohol Test With Oxidation.