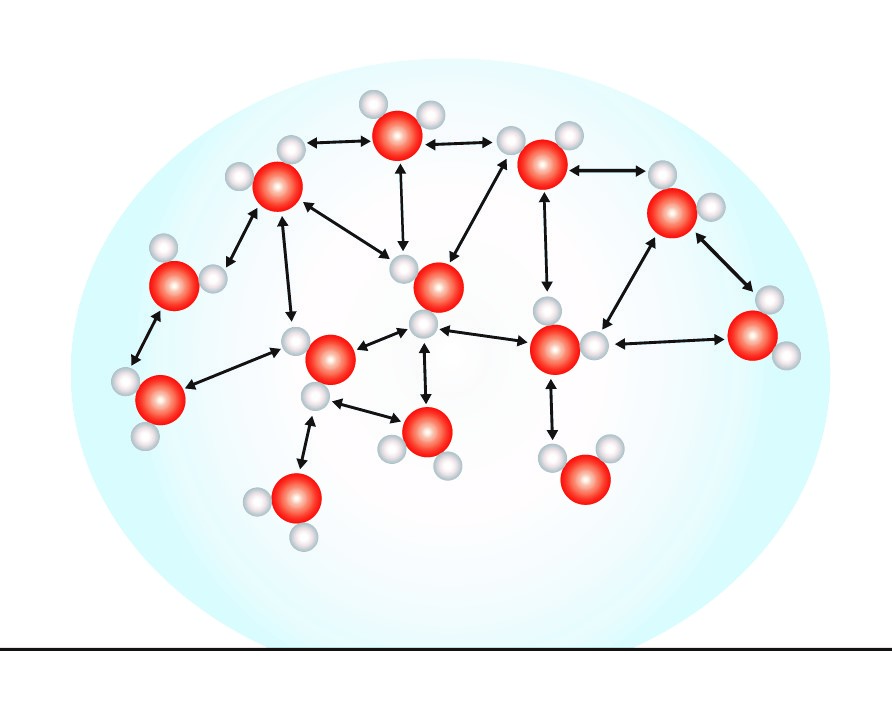
Surface Tension Organic Chemistry Tutor . Surface tension is a property of liquids that arises from the cohesive forces between the surface molecules, causing the surface of a. Chemistry coach has one idea in mind: Viscosity, cohesive and adhesive forces, surface tension, and capillary action. The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Surfactants like detergent), each solution exhibits differing surface tension properties. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Teach you everything you need to know about surface tension. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Gasoline) or solutes in the liquid (e.g. Allowing you to master general and. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
from www.acs.org
Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Gasoline) or solutes in the liquid (e.g. The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Surfactants like detergent), each solution exhibits differing surface tension properties. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Chemistry coach has one idea in mind: Allowing you to master general and. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension is a property of liquids that arises from the cohesive forces between the surface molecules, causing the surface of a. Teach you everything you need to know about surface tension.
Simulations & Videos for Lesson 5.2 Surface Tension American
Surface Tension Organic Chemistry Tutor Surfactants like detergent), each solution exhibits differing surface tension properties. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Allowing you to master general and. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Teach you everything you need to know about surface tension. Surfactants like detergent), each solution exhibits differing surface tension properties. The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Surface tension is a property of liquids that arises from the cohesive forces between the surface molecules, causing the surface of a. Viscosity, cohesive and adhesive forces, surface tension, and capillary action. Chemistry coach has one idea in mind: Gasoline) or solutes in the liquid (e.g. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
From www.purechemistry.org
SURFACE TENSION Purechemistry Surface Tension Organic Chemistry Tutor Since these intermolecular forces vary depending on the nature of the liquid (e.g. The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Gasoline) or solutes in the liquid (e.g. Allowing you to master general and. Surface tension is a property. Surface Tension Organic Chemistry Tutor.
From www.studypool.com
SOLUTION Surface chemistry surface tension and wet experiment Studypool Surface Tension Organic Chemistry Tutor Allowing you to master general and. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension is a property of liquids that arises from the cohesive forces between the surface molecules, causing the surface of a. Viscosity, cohesive and adhesive forces, surface tension,. Surface Tension Organic Chemistry Tutor.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces Surface Tension Organic Chemistry Tutor Surfactants like detergent), each solution exhibits differing surface tension properties. Allowing you to master general and. Viscosity, cohesive and adhesive forces, surface tension, and capillary action. The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Surface tension is a property of liquids that arises from the cohesive forces between the surface molecules,. Surface Tension Organic Chemistry Tutor.
From www.acs.org
Simulations & Videos for Lesson 5.2 Surface Tension American Surface Tension Organic Chemistry Tutor Teach you everything you need to know about surface tension. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Viscosity, cohesive and adhesive forces, surface tension, and capillary action. Surface tension is a property of liquids that arises from the cohesive forces between the. Surface Tension Organic Chemistry Tutor.
From www.etutorworld.com
Viscosity and Surface Tension eTutorWorld Surface Tension Organic Chemistry Tutor Surfactants like detergent), each solution exhibits differing surface tension properties. Teach you everything you need to know about surface tension. Surface tension is a property of liquids that arises from the cohesive forces between the surface molecules, causing the surface of a. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Allowing you to master general. Surface Tension Organic Chemistry Tutor.
From www.vedantu.com
States of Matter Chapter For JEE Main Chemistry Surface Tension Organic Chemistry Tutor The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Gasoline) or solutes in the liquid (e.g. Surface tension is a property of liquids that arises from the. Surface Tension Organic Chemistry Tutor.
From www.youtube.com
The Organic Chemistry Tutor Channel YouTube Surface Tension Organic Chemistry Tutor Surface tension is a property of liquids that arises from the cohesive forces between the surface molecules, causing the surface of a. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Liquids that have strong intermolecular. Surface Tension Organic Chemistry Tutor.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson Surface Tension Organic Chemistry Tutor Allowing you to master general and. Chemistry coach has one idea in mind: Surface tension is a property of liquids that arises from the cohesive forces between the surface molecules, causing the surface of a. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Gasoline) or solutes in the liquid (e.g. Viscosity, cohesive and adhesive forces, surface. Surface Tension Organic Chemistry Tutor.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) Surface Tension Organic Chemistry Tutor Chemistry coach has one idea in mind: Since these intermolecular forces vary depending on the nature of the liquid (e.g. The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Teach you everything you need to know about surface tension. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Allowing. Surface Tension Organic Chemistry Tutor.
From civilmint.com
What is Surface Tension Definition of Surface Tension, Examples and Test Surface Tension Organic Chemistry Tutor Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Teach you everything you need to know about surface tension. Chemistry coach has one idea in mind: Viscosity, cohesive and adhesive forces, surface tension, and capillary action. Since these intermolecular forces vary depending on the nature of the liquid (e.g.. Surface Tension Organic Chemistry Tutor.
From www.youtube.com
Surface tension States of matter and intermolecular forces Surface Tension Organic Chemistry Tutor Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Surface tension is a property of liquids that arises from the cohesive forces between the surface molecules, causing the surface of a. Surfactants like detergent), each solution exhibits differing surface tension properties. Gasoline) or solutes in the liquid (e.g. The surface tension of a liquid is a measure. Surface Tension Organic Chemistry Tutor.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Surface Tension Organic Chemistry Tutor Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Teach you everything you need to know about surface tension. Since these intermolecular forces vary depending on the nature of the liquid (e.g. The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Surface tension is the energy, or work, required. Surface Tension Organic Chemistry Tutor.
From www.pw.live
Surface Tension Surface Tension Organic Chemistry Tutor Chemistry coach has one idea in mind: Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Allowing you to. Surface Tension Organic Chemistry Tutor.
From 88guru.com
Viscosity And Surface Tension Definition, Applications and FAQ's 88Guru Surface Tension Organic Chemistry Tutor Teach you everything you need to know about surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Chemistry coach has one idea in mind: Gasoline) or solutes in the liquid (e.g. Surfactants like detergent), each solution exhibits differing surface tension properties. Liquids that have strong intermolecular forces,. Surface Tension Organic Chemistry Tutor.
From testbook.com
Surface Tension & Capillarity with Application, Formula & Examples Surface Tension Organic Chemistry Tutor Chemistry coach has one idea in mind: Surfactants like detergent), each solution exhibits differing surface tension properties. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Viscosity, cohesive and adhesive forces, surface tension, and capillary action.. Surface Tension Organic Chemistry Tutor.
From www.researchgate.net
Schematic illustration of standard methods of surface tension Surface Tension Organic Chemistry Tutor Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Chemistry coach has one idea in mind: Gasoline) or solutes in the liquid (e.g. Viscosity, cohesive and adhesive forces, surface tension, and capillary action. Since these intermolecular forces vary depending on the nature of the liquid (e.g. The surface tension. Surface Tension Organic Chemistry Tutor.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Surface Tension Organic Chemistry Tutor Chemistry coach has one idea in mind: Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Teach you everything you need to know about surface tension. Surface tension is a property of liquids that arises from the cohesive forces between the surface molecules, causing the surface of a. The. Surface Tension Organic Chemistry Tutor.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and Surface Tension Organic Chemistry Tutor Allowing you to master general and. The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Viscosity, cohesive and adhesive forces, surface tension, and capillary action. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Surface tension is the energy, or work, required to increase the surface area of a. Surface Tension Organic Chemistry Tutor.
From scienceinfo.com
Surface Tension Definition, Formula, Unit, Causes, Examples, Consequences Surface Tension Organic Chemistry Tutor Chemistry coach has one idea in mind: Teach you everything you need to know about surface tension. Gasoline) or solutes in the liquid (e.g. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surfactants like detergent), each solution exhibits differing surface tension properties. Allowing you to master general and. Viscosity, cohesive and adhesive forces, surface tension,. Surface Tension Organic Chemistry Tutor.
From www.tutordale.com
What Is Surface Tension In Chemistry Surface Tension Organic Chemistry Tutor Chemistry coach has one idea in mind: Surfactants like detergent), each solution exhibits differing surface tension properties. Allowing you to master general and. Viscosity, cohesive and adhesive forces, surface tension, and capillary action. Gasoline) or solutes in the liquid (e.g. Since these intermolecular forces vary depending on the nature of the liquid (e.g. The surface tension of a liquid is. Surface Tension Organic Chemistry Tutor.
From askfilo.com
\ 6 \rightarrow What is surface tension of liquids? Explain the effect o.. Surface Tension Organic Chemistry Tutor Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Viscosity, cohesive and adhesive forces, surface tension, and capillary action. Chemistry coach has one idea in mind: Surface tension is a property of liquids that arises from the cohesive forces between the surface molecules, causing the surface of a. Allowing you to master general and. Surface tension is. Surface Tension Organic Chemistry Tutor.
From www.mdpi.com
Predictive Model for the Surface Tension Changes of Chemical Solutions Surface Tension Organic Chemistry Tutor Surface tension is a property of liquids that arises from the cohesive forces between the surface molecules, causing the surface of a. Teach you everything you need to know about surface tension. Chemistry coach has one idea in mind: Gasoline) or solutes in the liquid (e.g. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Liquids. Surface Tension Organic Chemistry Tutor.
From knowfhysics.blogspot.com
What is surface tension? Surface Tension Organic Chemistry Tutor Gasoline) or solutes in the liquid (e.g. Viscosity, cohesive and adhesive forces, surface tension, and capillary action. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Chemistry coach has one idea in mind: Allowing you to master general and. Teach you everything you need to know about surface tension. Surface tension is the energy, or work, required. Surface Tension Organic Chemistry Tutor.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit Surface Tension Organic Chemistry Tutor Surface tension is a property of liquids that arises from the cohesive forces between the surface molecules, causing the surface of a. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Gasoline) or solutes in the liquid (e.g. Chemistry coach has one idea in mind: Since these intermolecular forces vary depending on the nature of the liquid. Surface Tension Organic Chemistry Tutor.
From readchemistry.com
Determination of Surface Tension Read Chemistry Surface Tension Organic Chemistry Tutor Chemistry coach has one idea in mind: Surfactants like detergent), each solution exhibits differing surface tension properties. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Teach you everything you need to know about surface tension. The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Since these intermolecular forces. Surface Tension Organic Chemistry Tutor.
From www.tutordale.com
What Is Surface Tension In Chemistry Surface Tension Organic Chemistry Tutor Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Gasoline) or solutes in the liquid (e.g. Surface tension is a property of liquids that arises from the. Surface Tension Organic Chemistry Tutor.
From www.slideserve.com
PPT Chapter 10 Liquids and Solids PowerPoint Presentation, free Surface Tension Organic Chemistry Tutor The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Chemistry coach has one idea in mind: Gasoline) or solutes in the liquid (e.g. Allowing you to master general and. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Teach you everything you need to know about surface tension.. Surface Tension Organic Chemistry Tutor.
From www.youtube.com
Chemistry 8.2b Properties of Liquids Surface Tension and Capillary Surface Tension Organic Chemistry Tutor Teach you everything you need to know about surface tension. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Allowing you to master general and. Chemistry coach has one idea in mind: Surface tension is the energy, or work, required. Surface Tension Organic Chemistry Tutor.
From www.esaral.com
Mind Maps for Fluid Surface Tension Revision Class 11, JEE, NEET Surface Tension Organic Chemistry Tutor Allowing you to master general and. Teach you everything you need to know about surface tension. Chemistry coach has one idea in mind: Viscosity, cohesive and adhesive forces, surface tension, and capillary action. Surface tension is a property of liquids that arises from the cohesive forces between the surface molecules, causing the surface of a. Liquids that have strong intermolecular. Surface Tension Organic Chemistry Tutor.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and Surface Tension Organic Chemistry Tutor Teach you everything you need to know about surface tension. The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Chemistry coach has one idea in mind: Surface tension is a property of liquids that arises from the cohesive forces between the surface molecules, causing the surface of a. Surface tension is the. Surface Tension Organic Chemistry Tutor.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Surface Tension Organic Chemistry Tutor Allowing you to master general and. Gasoline) or solutes in the liquid (e.g. Surface tension is a property of liquids that arises from the cohesive forces between the surface molecules, causing the surface of a. Surfactants like detergent), each solution exhibits differing surface tension properties. Chemistry coach has one idea in mind: Viscosity, cohesive and adhesive forces, surface tension, and. Surface Tension Organic Chemistry Tutor.
From classnotes.org.in
Surface Tension Chemistry, Class 11, States of Matter Surface Tension Organic Chemistry Tutor Teach you everything you need to know about surface tension. Surface tension is a property of liquids that arises from the cohesive forces between the surface molecules, causing the surface of a. Allowing you to master general and. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension is the energy, or work, required to. Surface Tension Organic Chemistry Tutor.
From www.vrogue.co
Surface Tension Chemistry Libretexts vrogue.co Surface Tension Organic Chemistry Tutor Allowing you to master general and. Viscosity, cohesive and adhesive forces, surface tension, and capillary action. The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Chemistry coach has one idea in mind: Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.. Surface Tension Organic Chemistry Tutor.
From www.researchgate.net
Schematic illustration of standard methods of surface tension Surface Tension Organic Chemistry Tutor Chemistry coach has one idea in mind: Surfactants like detergent), each solution exhibits differing surface tension properties. Teach you everything you need to know about surface tension. Since these intermolecular forces vary depending on the nature of the liquid (e.g. The surface tension of a liquid is a measure of the elastic force in the liquid’s surface. Allowing you to. Surface Tension Organic Chemistry Tutor.
From byjus.com
Explain the surface tension phenomenon with examples. Surface Tension Organic Chemistry Tutor Viscosity, cohesive and adhesive forces, surface tension, and capillary action. Allowing you to master general and. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Gasoline) or solutes in the liquid (e.g. Surfactants like detergent), each solution exhibits differing surface tension properties. Liquids that have strong intermolecular forces, like the hydrogen bonding in water,. Surface tension. Surface Tension Organic Chemistry Tutor.