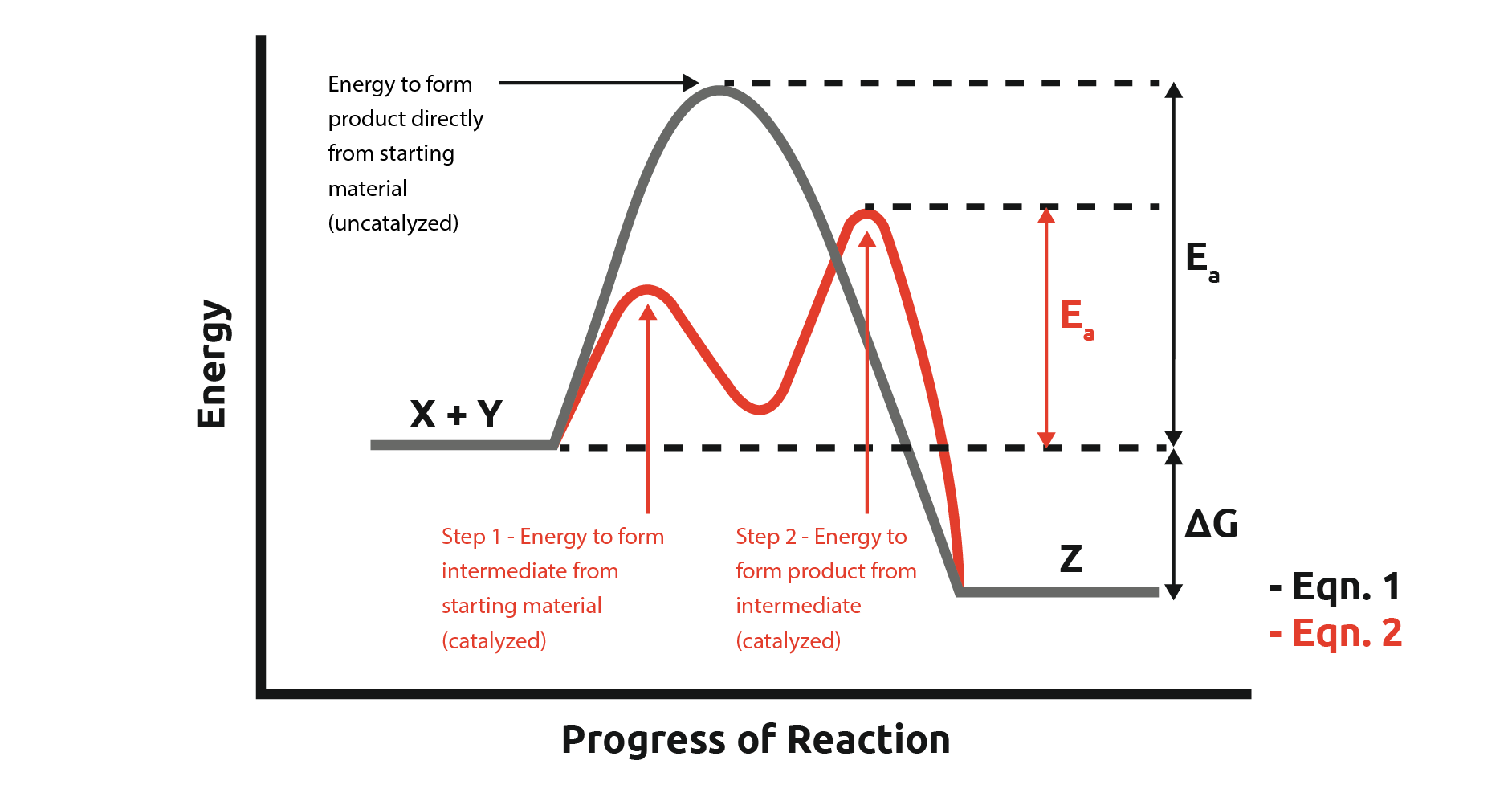
Catalyst Vs Cause . catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an. a catalyst speeds up the rate of a reaction by lowering the activation energy; a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. as nouns the difference between cause and catalyst is that cause is the source of, or reason for, an event or action; In addition, the catalyst is regenerated in the process. In homogeneous catalysis, catalysts are in the same phase as the reactants. — a catalyst accelerates a reaction without being consumed, focusing on chemical processes; In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption.
from www.labunlimited.com
as nouns the difference between cause and catalyst is that cause is the source of, or reason for, an event or action; In homogeneous catalysis, catalysts are in the same phase as the reactants. a catalyst speeds up the rate of a reaction by lowering the activation energy; catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. — a catalyst accelerates a reaction without being consumed, focusing on chemical processes; In addition, the catalyst is regenerated in the process. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an.
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited
Catalyst Vs Cause a catalyst speeds up the rate of a reaction by lowering the activation energy; — a catalyst accelerates a reaction without being consumed, focusing on chemical processes; Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. a catalyst speeds up the rate of a reaction by lowering the activation energy; as nouns the difference between cause and catalyst is that cause is the source of, or reason for, an event or action; In homogeneous catalysis, catalysts are in the same phase as the reactants. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. In addition, the catalyst is regenerated in the process.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalyst Vs Cause this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an. In homogeneous catalysis, catalysts are in the same phase as the reactants. In addition, the catalyst is regenerated in the process. — a catalyst accelerates a reaction without being consumed, focusing on chemical processes; a catalyst has no effect on. Catalyst Vs Cause.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Catalyst Vs Cause Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. this. Catalyst Vs Cause.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Catalyst Vs Cause — a catalyst accelerates a reaction without being consumed, focusing on chemical processes; In addition, the catalyst is regenerated in the process. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. a catalyst has no effect on the solution equilibrium of a reaction, it. Catalyst Vs Cause.
From schematicbraginamh.z4.web.core.windows.net
Energy Diagram For Chemical Reaction Catalyst Vs Cause a catalyst speeds up the rate of a reaction by lowering the activation energy; In homogeneous catalysis, catalysts are in the same phase as the reactants. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. this difference illustrates the means by which a catalyst. Catalyst Vs Cause.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Vs Cause Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an. a catalyst. Catalyst Vs Cause.
From thecontentauthority.com
Catalyst vs Catalase When To Use Each One? What To Consider Catalyst Vs Cause — a catalyst accelerates a reaction without being consumed, focusing on chemical processes; In homogeneous catalysis, catalysts are in the same phase as the reactants. a catalyst speeds up the rate of a reaction by lowering the activation energy; this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an. . Catalyst Vs Cause.
From www.globalspec.com
Catalysts and Initiators Information Engineering360 Catalyst Vs Cause catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. . Catalyst Vs Cause.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Vs Cause a catalyst speeds up the rate of a reaction by lowering the activation energy; Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. this difference illustrates the means by. Catalyst Vs Cause.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Vs Cause as nouns the difference between cause and catalyst is that cause is the source of, or reason for, an event or action; Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. catalysts have no effect on the equilibrium constant and thus on the equilibrium. Catalyst Vs Cause.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Vs Cause a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. catalysts allow a reaction to proceed via a pathway that has a lower activation energy. Catalyst Vs Cause.
From study.com
Effect of Catalysts on Rates of Reaction Lesson Catalyst Vs Cause Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. . Catalyst Vs Cause.
From www.slideserve.com
PPT Chapter 2Enzymes PowerPoint Presentation, free download ID5566824 Catalyst Vs Cause catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. as nouns the difference between cause and catalyst is that cause is the source of, or reason for, an event or. Catalyst Vs Cause.
From www.researchgate.net
Homogeneous vs heterogeneous catalysts. Design of heterogeneous Catalyst Vs Cause In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium.. Catalyst Vs Cause.
From slideplayer.com
Reaction Mechanisms ppt download Catalyst Vs Cause In addition, the catalyst is regenerated in the process. In homogeneous catalysis, catalysts are in the same phase as the reactants. a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. — a catalyst accelerates a reaction without being consumed, focusing on chemical processes; catalysts allow a. Catalyst Vs Cause.
From www.slideserve.com
PPT Factors that Affect Rate of Reaction PowerPoint Presentation Catalyst Vs Cause catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. as nouns the difference between cause and catalyst is that cause is the source of, or reason for, an event or action; . Catalyst Vs Cause.
From www.savemyexams.com
Types of Catalyst CIE A Level Chemistry Revision Notes 2025 Catalyst Vs Cause — a catalyst accelerates a reaction without being consumed, focusing on chemical processes; In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. as nouns the difference between cause and catalyst is that cause is the source of, or reason for, an event or action; a catalyst speeds up the rate. Catalyst Vs Cause.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Vs Cause as nouns the difference between cause and catalyst is that cause is the source of, or reason for, an event or action; catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable. Catalyst Vs Cause.
From fr.slideserve.com
PPT 15 Chemical PowerPoint Presentation, free download ID Catalyst Vs Cause In homogeneous catalysis, catalysts are in the same phase as the reactants. this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an. In addition, the catalyst is regenerated in the process. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. . Catalyst Vs Cause.
From www.youtube.com
Cause vs Catalyst YouTube Catalyst Vs Cause In addition, the catalyst is regenerated in the process. this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an. In homogeneous catalysis, catalysts are in the same phase as the reactants. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. a catalyst speeds up. Catalyst Vs Cause.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Catalyst Vs Cause In addition, the catalyst is regenerated in the process. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. In heterogeneous catalysis, catalysts provide a surface to which reactants. Catalyst Vs Cause.
From www.slideserve.com
PPT Homogeneous Catalysis Introduction PowerPoint Presentation Catalyst Vs Cause — a catalyst accelerates a reaction without being consumed, focusing on chemical processes; Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. In homogeneous catalysis, catalysts are in the same phase as the reactants. catalysts allow a reaction to proceed via a pathway that. Catalyst Vs Cause.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Vs Cause this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing an. catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. Several reactions that are thermodynamically favorable. Catalyst Vs Cause.
From ammoniaknowhow.com
Catalyst deactivation Common causes AmmoniaKnowHow Catalyst Vs Cause a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present.. Catalyst Vs Cause.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Catalyst Vs Cause a catalyst speeds up the rate of a reaction by lowering the activation energy; Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. In addition, the catalyst is regenerated in the process. — a catalyst accelerates a reaction without being consumed, focusing on chemical. Catalyst Vs Cause.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalyst Vs Cause a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. catalysts allow a reaction to proceed via. Catalyst Vs Cause.
From www.pinterest.co.uk
Catalyst and Mechanism of Enzyme Catalysis Enzymes, Chemistry, Active Catalyst Vs Cause catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. as nouns the difference between cause and catalyst is that cause is the source of, or reason for, an event or action; this difference illustrates the means by which a catalyst functions to accelerate reactions, namely, by providing. Catalyst Vs Cause.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Vs Cause — a catalyst accelerates a reaction without being consumed, focusing on chemical processes; catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. as nouns the difference between cause and catalyst is that cause is the source of, or reason for, an event or action; Several reactions that are thermodynamically favorable in the. Catalyst Vs Cause.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Vs Cause a catalyst speeds up the rate of a reaction by lowering the activation energy; as nouns the difference between cause and catalyst is that cause is the source of, or reason for, an event or action; — a catalyst accelerates a reaction without being consumed, focusing on chemical processes; In heterogeneous catalysis, catalysts provide a surface to. Catalyst Vs Cause.
From www.slideserve.com
PPT Chapter 17 Reaction PowerPoint Presentation, free Catalyst Vs Cause — a catalyst accelerates a reaction without being consumed, focusing on chemical processes; Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. this difference. Catalyst Vs Cause.
From www.differencebetween.com
Difference Between Catalyst and Reagent Compare the Difference Catalyst Vs Cause In homogeneous catalysis, catalysts are in the same phase as the reactants. In addition, the catalyst is regenerated in the process. catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur at a reasonable rate when a catalyst is present. . Catalyst Vs Cause.
From nic-ibchem.blogspot.com
IB Chemistry (HL) B7 Enzymes Catalyst Vs Cause catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to which reactants bind in a process of adsorption. In addition, the catalyst is regenerated in the process.. Catalyst Vs Cause.
From thecontentauthority.com
Catalyst vs Cause When To Use Each One? What To Consider Catalyst Vs Cause — a catalyst accelerates a reaction without being consumed, focusing on chemical processes; a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. In homogeneous catalysis, catalysts are in the same phase as the reactants. In addition, the catalyst is regenerated in the process. as nouns the. Catalyst Vs Cause.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Vs Cause catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. as nouns the difference between cause and catalyst is that cause is the source of, or reason for, an event or action; — a catalyst accelerates a reaction without being consumed, focusing on chemical processes; this difference. Catalyst Vs Cause.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Vs Cause In homogeneous catalysis, catalysts are in the same phase as the reactants. catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. a catalyst has no effect on the solution equilibrium of a reaction, it increases the rate of approach to equilibrium. a catalyst speeds up the rate. Catalyst Vs Cause.
From www.youtube.com
B.7.2 Compare catalysts and biological catalysts (enzymes Catalyst Vs Cause catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. — a catalyst accelerates a reaction without being consumed, focusing on chemical processes; In homogeneous catalysis, catalysts are in the same phase as the reactants. Several reactions that are thermodynamically favorable in the absence of a catalyst only occur. Catalyst Vs Cause.