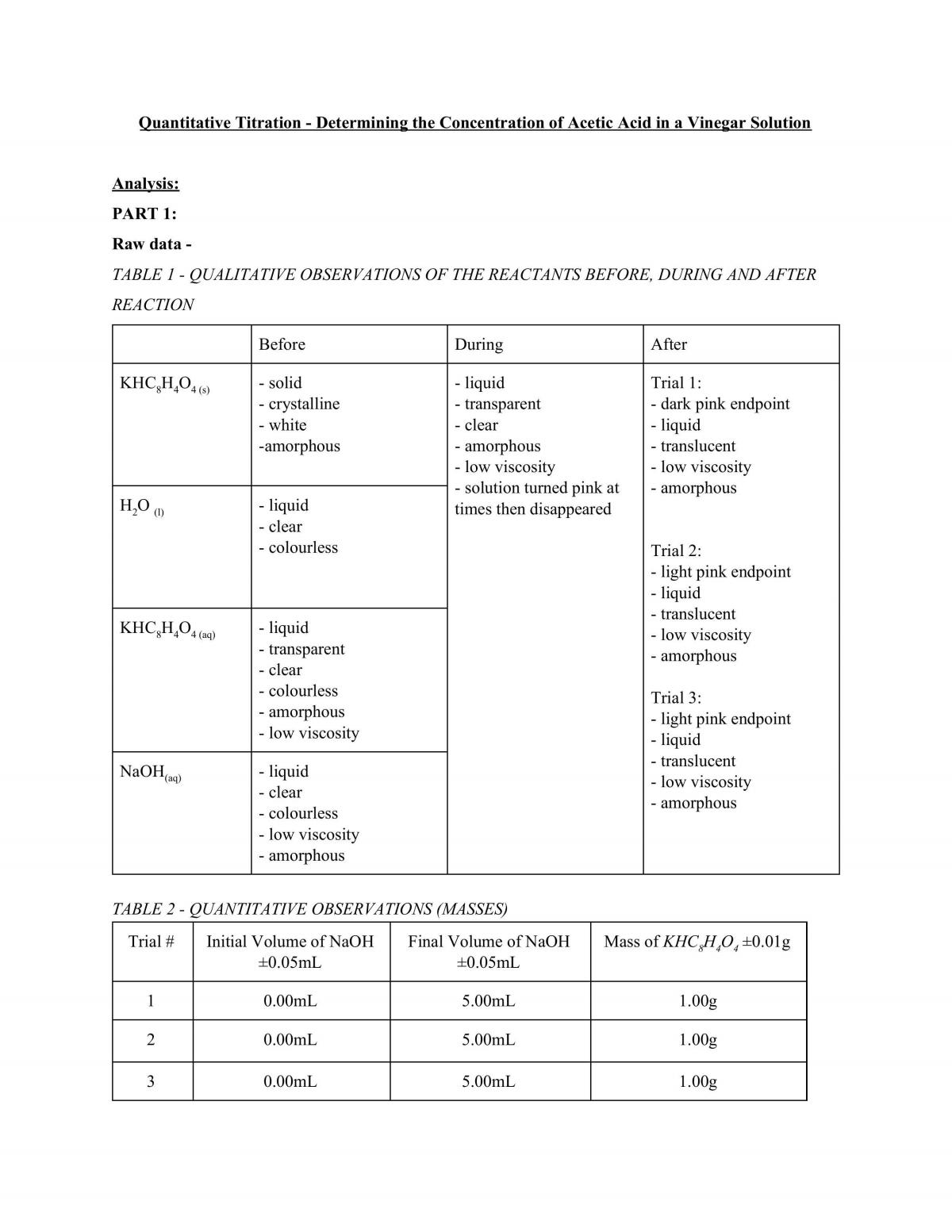
Lab Normality Of Acetic Acid . Know the normality formula, equations, tips to calculate normality, its relationship. lab session 7, experiment 6: Preparation of 1m acetic acid solution from glacial acetic acid. a 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. Molarity (m) and normality (n) are two means of expressing solute. 14 rows molarity of concentrated acids & bases. The density of glacial acetic acid is 1.049 g/ml at 25°c. The purpose of this experiment is to prepare and to standardize secondary standard solution; Dilutions to make a 1 molar solution. normality is used to measure the concentration of a solution.
from www.thinkswap.com
Dilutions to make a 1 molar solution. The density of glacial acetic acid is 1.049 g/ml at 25°c. The purpose of this experiment is to prepare and to standardize secondary standard solution; 14 rows molarity of concentrated acids & bases. lab session 7, experiment 6: normality is used to measure the concentration of a solution. a 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. Know the normality formula, equations, tips to calculate normality, its relationship. Molarity (m) and normality (n) are two means of expressing solute. Preparation of 1m acetic acid solution from glacial acetic acid.
Concentration of Acetic Acid Chemistry (University) Grade 11 OSSD
Lab Normality Of Acetic Acid Molarity (m) and normality (n) are two means of expressing solute. 14 rows molarity of concentrated acids & bases. a 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. Dilutions to make a 1 molar solution. Know the normality formula, equations, tips to calculate normality, its relationship. lab session 7, experiment 6: normality is used to measure the concentration of a solution. The density of glacial acetic acid is 1.049 g/ml at 25°c. Preparation of 1m acetic acid solution from glacial acetic acid. The purpose of this experiment is to prepare and to standardize secondary standard solution; Molarity (m) and normality (n) are two means of expressing solute.
From laboratorytalk.com
Anton Paar discusses measurement of acetic acid Laboratory Talk Lab Normality Of Acetic Acid Preparation of 1m acetic acid solution from glacial acetic acid. Dilutions to make a 1 molar solution. Molarity (m) and normality (n) are two means of expressing solute. normality is used to measure the concentration of a solution. The purpose of this experiment is to prepare and to standardize secondary standard solution; lab session 7, experiment 6: Know. Lab Normality Of Acetic Acid.
From snipe.fm
😍 Percentage of acetic acid in vinegar lab. Determination of Acetic Lab Normality Of Acetic Acid lab session 7, experiment 6: normality is used to measure the concentration of a solution. 14 rows molarity of concentrated acids & bases. The density of glacial acetic acid is 1.049 g/ml at 25°c. The purpose of this experiment is to prepare and to standardize secondary standard solution; Preparation of 1m acetic acid solution from glacial acetic. Lab Normality Of Acetic Acid.
From studylib.net
Acetic Acid with Baking Soda Lab Lab Normality Of Acetic Acid Molarity (m) and normality (n) are two means of expressing solute. Preparation of 1m acetic acid solution from glacial acetic acid. The density of glacial acetic acid is 1.049 g/ml at 25°c. Know the normality formula, equations, tips to calculate normality, its relationship. The purpose of this experiment is to prepare and to standardize secondary standard solution; Dilutions to make. Lab Normality Of Acetic Acid.
From pharmabeej.com
How To Prepare 1M Acetic Acid In Pharma? Pharmabeej Lab Normality Of Acetic Acid normality is used to measure the concentration of a solution. Molarity (m) and normality (n) are two means of expressing solute. Know the normality formula, equations, tips to calculate normality, its relationship. The density of glacial acetic acid is 1.049 g/ml at 25°c. Dilutions to make a 1 molar solution. lab session 7, experiment 6: The purpose of. Lab Normality Of Acetic Acid.
From byjus.com
The normality of 100 Lab Normality Of Acetic Acid Dilutions to make a 1 molar solution. normality is used to measure the concentration of a solution. lab session 7, experiment 6: Know the normality formula, equations, tips to calculate normality, its relationship. Molarity (m) and normality (n) are two means of expressing solute. The density of glacial acetic acid is 1.049 g/ml at 25°c. a 99.7%. Lab Normality Of Acetic Acid.
From www.numerade.com
SOLVED REPORT SHEET AcidBase Titration LAB 20 Concentration of Acetic Lab Normality Of Acetic Acid The purpose of this experiment is to prepare and to standardize secondary standard solution; Dilutions to make a 1 molar solution. normality is used to measure the concentration of a solution. Preparation of 1m acetic acid solution from glacial acetic acid. The density of glacial acetic acid is 1.049 g/ml at 25°c. 14 rows molarity of concentrated acids. Lab Normality Of Acetic Acid.
From askfilo.com
Normality of 10(w/v) acetic acid solution is Filo Lab Normality Of Acetic Acid normality is used to measure the concentration of a solution. Dilutions to make a 1 molar solution. a 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. Preparation of 1m acetic acid solution from glacial acetic acid. Molarity (m) and normality (n) are two means of expressing solute.. Lab Normality Of Acetic Acid.
From www.youtube.com
, The normality of 10 (weight/volume) acetic acid is(1) 1 N (2) 10 N Lab Normality Of Acetic Acid normality is used to measure the concentration of a solution. Preparation of 1m acetic acid solution from glacial acetic acid. The purpose of this experiment is to prepare and to standardize secondary standard solution; Dilutions to make a 1 molar solution. The density of glacial acetic acid is 1.049 g/ml at 25°c. 14 rows molarity of concentrated acids. Lab Normality Of Acetic Acid.
From www.coursehero.com
[Solved] the molarity of acetic acid. Lab Data X Didi our data to the Lab Normality Of Acetic Acid Dilutions to make a 1 molar solution. Molarity (m) and normality (n) are two means of expressing solute. The density of glacial acetic acid is 1.049 g/ml at 25°c. Know the normality formula, equations, tips to calculate normality, its relationship. lab session 7, experiment 6: The purpose of this experiment is to prepare and to standardize secondary standard solution;. Lab Normality Of Acetic Acid.
From www.numerade.com
SOLVEDCalculate the normality of each of the following solutions. a. 0 Lab Normality Of Acetic Acid lab session 7, experiment 6: Dilutions to make a 1 molar solution. Molarity (m) and normality (n) are two means of expressing solute. Preparation of 1m acetic acid solution from glacial acetic acid. The purpose of this experiment is to prepare and to standardize secondary standard solution; 14 rows molarity of concentrated acids & bases. a 99.7%. Lab Normality Of Acetic Acid.
From www.chemistry-online.com
Lewis structure of acetic acid CH3COOH Chemistry Online Lab Normality Of Acetic Acid Preparation of 1m acetic acid solution from glacial acetic acid. Dilutions to make a 1 molar solution. The density of glacial acetic acid is 1.049 g/ml at 25°c. Molarity (m) and normality (n) are two means of expressing solute. normality is used to measure the concentration of a solution. Know the normality formula, equations, tips to calculate normality, its. Lab Normality Of Acetic Acid.
From www.doubtnut.com
The normality of 10 ( weight // volume ) acetic acid is Lab Normality Of Acetic Acid normality is used to measure the concentration of a solution. Dilutions to make a 1 molar solution. Molarity (m) and normality (n) are two means of expressing solute. The density of glacial acetic acid is 1.049 g/ml at 25°c. Know the normality formula, equations, tips to calculate normality, its relationship. Preparation of 1m acetic acid solution from glacial acetic. Lab Normality Of Acetic Acid.
From www.youtube.com
1 normal Acetic acid 1 N acetic acid YouTube Lab Normality Of Acetic Acid a 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. The density of glacial acetic acid is 1.049 g/ml at 25°c. Molarity (m) and normality (n) are two means of expressing solute. lab session 7, experiment 6: 14 rows molarity of concentrated acids & bases. Preparation of. Lab Normality Of Acetic Acid.
From printable.mapadapalavra.ba.gov.br
Normal Lab Values Chart Printable Lab Normality Of Acetic Acid Molarity (m) and normality (n) are two means of expressing solute. 14 rows molarity of concentrated acids & bases. Preparation of 1m acetic acid solution from glacial acetic acid. Dilutions to make a 1 molar solution. a 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. The density. Lab Normality Of Acetic Acid.
From www.thoughtco.com
How to Calculate Normality of a Solution Lab Normality Of Acetic Acid 14 rows molarity of concentrated acids & bases. Molarity (m) and normality (n) are two means of expressing solute. Preparation of 1m acetic acid solution from glacial acetic acid. normality is used to measure the concentration of a solution. The purpose of this experiment is to prepare and to standardize secondary standard solution; lab session 7, experiment. Lab Normality Of Acetic Acid.
From www.studocu.com
AcidBase Titration Lab Part II Determination of Citric Acid in Lab Normality Of Acetic Acid Preparation of 1m acetic acid solution from glacial acetic acid. The purpose of this experiment is to prepare and to standardize secondary standard solution; The density of glacial acetic acid is 1.049 g/ml at 25°c. normality is used to measure the concentration of a solution. 14 rows molarity of concentrated acids & bases. Dilutions to make a 1. Lab Normality Of Acetic Acid.
From www.numerade.com
SOLVED Acetic acid has a normal boiling point of 118 ∘C and a ΔHvap of Lab Normality Of Acetic Acid Dilutions to make a 1 molar solution. lab session 7, experiment 6: normality is used to measure the concentration of a solution. The density of glacial acetic acid is 1.049 g/ml at 25°c. Molarity (m) and normality (n) are two means of expressing solute. 14 rows molarity of concentrated acids & bases. a 99.7% (w/w) glacial. Lab Normality Of Acetic Acid.
From www.numerade.com
SOLVED Q5/ In the titration of NaOH solution and acetic acid solution Lab Normality Of Acetic Acid lab session 7, experiment 6: The purpose of this experiment is to prepare and to standardize secondary standard solution; Preparation of 1m acetic acid solution from glacial acetic acid. 14 rows molarity of concentrated acids & bases. The density of glacial acetic acid is 1.049 g/ml at 25°c. normality is used to measure the concentration of a. Lab Normality Of Acetic Acid.
From www.numerade.com
SOLVED REPORT SHEET LAB AcidBase Titration 20 Concentration of Acetic Lab Normality Of Acetic Acid 14 rows molarity of concentrated acids & bases. The density of glacial acetic acid is 1.049 g/ml at 25°c. Preparation of 1m acetic acid solution from glacial acetic acid. Know the normality formula, equations, tips to calculate normality, its relationship. Molarity (m) and normality (n) are two means of expressing solute. Dilutions to make a 1 molar solution. . Lab Normality Of Acetic Acid.
From www.carolina.com
Acetic Acid, 6 M (36 v/v), Laboratory Grade, 500 mL Carolina Lab Normality Of Acetic Acid Know the normality formula, equations, tips to calculate normality, its relationship. The purpose of this experiment is to prepare and to standardize secondary standard solution; Molarity (m) and normality (n) are two means of expressing solute. Preparation of 1m acetic acid solution from glacial acetic acid. normality is used to measure the concentration of a solution. a 99.7%. Lab Normality Of Acetic Acid.
From www.numerade.com
SOLVEDCalculate the normality of each of the following solutions. a. 0 Lab Normality Of Acetic Acid Know the normality formula, equations, tips to calculate normality, its relationship. Preparation of 1m acetic acid solution from glacial acetic acid. The density of glacial acetic acid is 1.049 g/ml at 25°c. 14 rows molarity of concentrated acids & bases. lab session 7, experiment 6: Dilutions to make a 1 molar solution. Molarity (m) and normality (n) are. Lab Normality Of Acetic Acid.
From exommzgkk.blob.core.windows.net
Medical Lab Test Values at Tony Pelletier blog Lab Normality Of Acetic Acid 14 rows molarity of concentrated acids & bases. normality is used to measure the concentration of a solution. Dilutions to make a 1 molar solution. a 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. The purpose of this experiment is to prepare and to standardize secondary. Lab Normality Of Acetic Acid.
From present5.com
Colposcopy Acetic acid test (3 5 acetic acid) Lab Normality Of Acetic Acid 14 rows molarity of concentrated acids & bases. normality is used to measure the concentration of a solution. The density of glacial acetic acid is 1.049 g/ml at 25°c. Molarity (m) and normality (n) are two means of expressing solute. a 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g. Lab Normality Of Acetic Acid.
From www.studocu.com
Lab Report Determination of Acetic Acid LAB REPORT DETERMINATION OF Lab Normality Of Acetic Acid Preparation of 1m acetic acid solution from glacial acetic acid. lab session 7, experiment 6: Molarity (m) and normality (n) are two means of expressing solute. a 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. 14 rows molarity of concentrated acids & bases. normality is. Lab Normality Of Acetic Acid.
From www.youtube.com
The normality of 10 (weightvolume) acetic acid is Class 11 Lab Normality Of Acetic Acid Know the normality formula, equations, tips to calculate normality, its relationship. Preparation of 1m acetic acid solution from glacial acetic acid. Dilutions to make a 1 molar solution. Molarity (m) and normality (n) are two means of expressing solute. normality is used to measure the concentration of a solution. 14 rows molarity of concentrated acids & bases. . Lab Normality Of Acetic Acid.
From www.formsbirds.com
Normal Lab Values Table Free Download Lab Normality Of Acetic Acid Preparation of 1m acetic acid solution from glacial acetic acid. 14 rows molarity of concentrated acids & bases. The purpose of this experiment is to prepare and to standardize secondary standard solution; a 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. normality is used to measure. Lab Normality Of Acetic Acid.
From www.wikidoc.org
Acetic acid wikidoc Lab Normality Of Acetic Acid Know the normality formula, equations, tips to calculate normality, its relationship. The purpose of this experiment is to prepare and to standardize secondary standard solution; a 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. Preparation of 1m acetic acid solution from glacial acetic acid. Dilutions to make a. Lab Normality Of Acetic Acid.
From www.toppr.com
Which of the following will represent the normality of 10 (w/V) acetic Lab Normality Of Acetic Acid lab session 7, experiment 6: Dilutions to make a 1 molar solution. 14 rows molarity of concentrated acids & bases. a 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. The purpose of this experiment is to prepare and to standardize secondary standard solution; Preparation of 1m. Lab Normality Of Acetic Acid.
From www.chegg.com
Solved Titration of Acetic Acid Titration of Acetic Acid in Lab Normality Of Acetic Acid Dilutions to make a 1 molar solution. The purpose of this experiment is to prepare and to standardize secondary standard solution; lab session 7, experiment 6: Know the normality formula, equations, tips to calculate normality, its relationship. Molarity (m) and normality (n) are two means of expressing solute. 14 rows molarity of concentrated acids & bases. The density. Lab Normality Of Acetic Acid.
From jonesthelf2002.blogspot.com
Easy Way to Learn Nursing Lab Values Jones Thelf2002 Lab Normality Of Acetic Acid The density of glacial acetic acid is 1.049 g/ml at 25°c. normality is used to measure the concentration of a solution. The purpose of this experiment is to prepare and to standardize secondary standard solution; Molarity (m) and normality (n) are two means of expressing solute. Dilutions to make a 1 molar solution. Preparation of 1m acetic acid solution. Lab Normality Of Acetic Acid.
From www.thinkswap.com
Concentration of Acetic Acid Chemistry (University) Grade 11 OSSD Lab Normality Of Acetic Acid a 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. The purpose of this experiment is to prepare and to standardize secondary standard solution; Molarity (m) and normality (n) are two means of expressing solute. Preparation of 1m acetic acid solution from glacial acetic acid. lab session 7,. Lab Normality Of Acetic Acid.
From www.coursehero.com
[Solved] Inall 2 Mass of acetic acid Density of acetic acid 1.00 g/mL Lab Normality Of Acetic Acid lab session 7, experiment 6: a 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. Dilutions to make a 1 molar solution. The density of glacial acetic acid is 1.049 g/ml at 25°c. normality is used to measure the concentration of a solution. Know the normality formula,. Lab Normality Of Acetic Acid.
From www.studypool.com
SOLUTION C Lab 6 Titration Of Acetic Acid In Vinegar Studypool Lab Normality Of Acetic Acid The purpose of this experiment is to prepare and to standardize secondary standard solution; Preparation of 1m acetic acid solution from glacial acetic acid. a 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. normality is used to measure the concentration of a solution. Molarity (m) and normality. Lab Normality Of Acetic Acid.
From studylib.net
Determining the Ka of Acetic Acid Lab Normality Of Acetic Acid The purpose of this experiment is to prepare and to standardize secondary standard solution; Dilutions to make a 1 molar solution. a 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. 14 rows molarity of concentrated acids & bases. normality is used to measure the concentration of. Lab Normality Of Acetic Acid.
From studylib.net
Acetic Acid Determination Lab Document Lab Normality Of Acetic Acid normality is used to measure the concentration of a solution. The purpose of this experiment is to prepare and to standardize secondary standard solution; a 99.7% (w/w) glacial acetic acid means that 100 g of glacial acetic acid contains 99.7 g of acetic acid. Preparation of 1m acetic acid solution from glacial acetic acid. Dilutions to make a. Lab Normality Of Acetic Acid.