What Is Dry Vapor Pressure Equivalent . a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. the result of astm d5191 is the dry vapor pressure equivalent, or dvpe. if v/l = 0, the vapor pressure is essentially equivalent to the bubble point of the mixture which is the highest vapor pressure value for the. Then in 1999, the triple expansion method for vapor. hello, could anyone please explain to me is there any difference between reid vapor pressure (rvp) and. this test method is suitable for testing samples with boiling points above 0 °c (32 °f) that exert a vapor pressure. rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or more simply called vapor pressure, is the vapor. the reid vapor pressure (rvp) can differ substantially from the true vapor pressure (tvp) of a liquid mixture, since (1) rvp is.
from www.numerade.com
a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. this test method is suitable for testing samples with boiling points above 0 °c (32 °f) that exert a vapor pressure. if v/l = 0, the vapor pressure is essentially equivalent to the bubble point of the mixture which is the highest vapor pressure value for the. hello, could anyone please explain to me is there any difference between reid vapor pressure (rvp) and. Then in 1999, the triple expansion method for vapor. the reid vapor pressure (rvp) can differ substantially from the true vapor pressure (tvp) of a liquid mixture, since (1) rvp is. rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or more simply called vapor pressure, is the vapor. the result of astm d5191 is the dry vapor pressure equivalent, or dvpe.
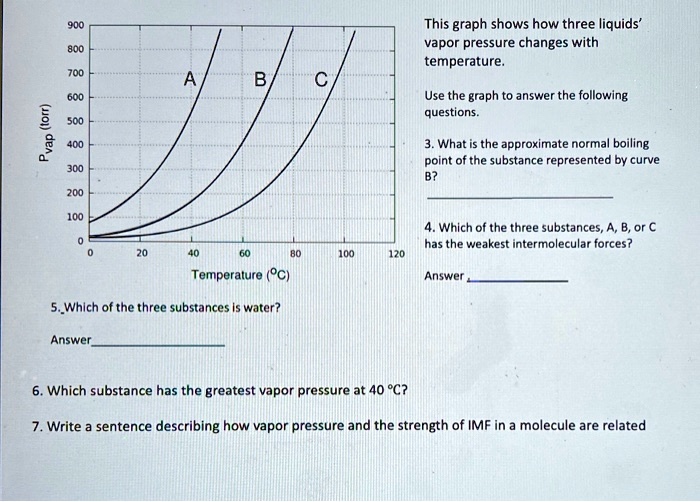
SOLVED This graph shows how three liquids' vapor pressure changes with
What Is Dry Vapor Pressure Equivalent hello, could anyone please explain to me is there any difference between reid vapor pressure (rvp) and. the reid vapor pressure (rvp) can differ substantially from the true vapor pressure (tvp) of a liquid mixture, since (1) rvp is. Then in 1999, the triple expansion method for vapor. if v/l = 0, the vapor pressure is essentially equivalent to the bubble point of the mixture which is the highest vapor pressure value for the. the result of astm d5191 is the dry vapor pressure equivalent, or dvpe. a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. hello, could anyone please explain to me is there any difference between reid vapor pressure (rvp) and. this test method is suitable for testing samples with boiling points above 0 °c (32 °f) that exert a vapor pressure. rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or more simply called vapor pressure, is the vapor.
From users.highland.edu
Vapor Pressure What Is Dry Vapor Pressure Equivalent if v/l = 0, the vapor pressure is essentially equivalent to the bubble point of the mixture which is the highest vapor pressure value for the. the reid vapor pressure (rvp) can differ substantially from the true vapor pressure (tvp) of a liquid mixture, since (1) rvp is. hello, could anyone please explain to me is there. What Is Dry Vapor Pressure Equivalent.
From www.slideserve.com
PPT Vapor and it Pressure PowerPoint Presentation, free download ID What Is Dry Vapor Pressure Equivalent hello, could anyone please explain to me is there any difference between reid vapor pressure (rvp) and. a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. this test method is suitable for testing samples with boiling points above 0 °c (32 °f) that exert a vapor pressure. the. What Is Dry Vapor Pressure Equivalent.
From jmpcoblog.com
How To Read A Pump Curve Part 2 What Is Dry Vapor Pressure Equivalent Then in 1999, the triple expansion method for vapor. this test method is suitable for testing samples with boiling points above 0 °c (32 °f) that exert a vapor pressure. a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. rvp, or what is more properly called the dry vapor. What Is Dry Vapor Pressure Equivalent.
From www.youtube.com
Vapor pressure versus temperature relations of common elements YouTube What Is Dry Vapor Pressure Equivalent rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or more simply called vapor pressure, is the vapor. the result of astm d5191 is the dry vapor pressure equivalent, or dvpe. the reid vapor pressure (rvp) can differ substantially from the true vapor pressure (tvp) of a liquid mixture, since (1) rvp is.. What Is Dry Vapor Pressure Equivalent.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 What Is Dry Vapor Pressure Equivalent the result of astm d5191 is the dry vapor pressure equivalent, or dvpe. Then in 1999, the triple expansion method for vapor. if v/l = 0, the vapor pressure is essentially equivalent to the bubble point of the mixture which is the highest vapor pressure value for the. hello, could anyone please explain to me is there. What Is Dry Vapor Pressure Equivalent.
From engineerexcel.com
Vapor Pressure of Water Explained EngineerExcel What Is Dry Vapor Pressure Equivalent Then in 1999, the triple expansion method for vapor. hello, could anyone please explain to me is there any difference between reid vapor pressure (rvp) and. a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. the reid vapor pressure (rvp) can differ substantially from the true vapor pressure (tvp). What Is Dry Vapor Pressure Equivalent.
From www.slideserve.com
PPT Vapor and it Pressure PowerPoint Presentation, free download ID What Is Dry Vapor Pressure Equivalent if v/l = 0, the vapor pressure is essentially equivalent to the bubble point of the mixture which is the highest vapor pressure value for the. this test method is suitable for testing samples with boiling points above 0 °c (32 °f) that exert a vapor pressure. a dry vapour pressure equivalent (dvpe) can be calculated from. What Is Dry Vapor Pressure Equivalent.
From webmis.highland.cc.il.us
Vapor Pressure What Is Dry Vapor Pressure Equivalent Then in 1999, the triple expansion method for vapor. a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or more simply called vapor pressure, is the vapor. the result of astm d5191 is the dry vapor. What Is Dry Vapor Pressure Equivalent.
From www.youtube.com
What is vapor pressure what is the vapor pressure of liquids What Is Dry Vapor Pressure Equivalent the reid vapor pressure (rvp) can differ substantially from the true vapor pressure (tvp) of a liquid mixture, since (1) rvp is. rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or more simply called vapor pressure, is the vapor. a dry vapour pressure equivalent (dvpe) can be calculated from the air containing. What Is Dry Vapor Pressure Equivalent.
From www.youtube.com
CHEMISTRY 201 Using the ClausiusClapeyron equation to solve for vapor What Is Dry Vapor Pressure Equivalent Then in 1999, the triple expansion method for vapor. a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. the reid vapor pressure (rvp) can differ substantially from the true vapor pressure (tvp) of a liquid mixture, since (1) rvp is. hello, could anyone please explain to me is there. What Is Dry Vapor Pressure Equivalent.
From www.en-standard.eu
UNE EN 1301612018 Liquid petroleum products Vapour pressure Part What Is Dry Vapor Pressure Equivalent the result of astm d5191 is the dry vapor pressure equivalent, or dvpe. Then in 1999, the triple expansion method for vapor. a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or more simply called vapor. What Is Dry Vapor Pressure Equivalent.
From drlogy.com
Best Pressure Conversion Calculator Pressure Units Drlogy What Is Dry Vapor Pressure Equivalent rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or more simply called vapor pressure, is the vapor. a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. the reid vapor pressure (rvp) can differ substantially from the true vapor pressure (tvp) of a liquid mixture,. What Is Dry Vapor Pressure Equivalent.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is Dry Vapor Pressure Equivalent Then in 1999, the triple expansion method for vapor. if v/l = 0, the vapor pressure is essentially equivalent to the bubble point of the mixture which is the highest vapor pressure value for the. this test method is suitable for testing samples with boiling points above 0 °c (32 °f) that exert a vapor pressure. the. What Is Dry Vapor Pressure Equivalent.
From www.en-standard.eu
BS EN 1301632018 Liquid petroleum products. Vapour pressure What Is Dry Vapor Pressure Equivalent Then in 1999, the triple expansion method for vapor. hello, could anyone please explain to me is there any difference between reid vapor pressure (rvp) and. a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or. What Is Dry Vapor Pressure Equivalent.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is Dry Vapor Pressure Equivalent rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or more simply called vapor pressure, is the vapor. the result of astm d5191 is the dry vapor pressure equivalent, or dvpe. a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. Then in 1999, the triple. What Is Dry Vapor Pressure Equivalent.
From www.slideserve.com
PPT 6 Gases PowerPoint Presentation, free download ID352441 What Is Dry Vapor Pressure Equivalent the result of astm d5191 is the dry vapor pressure equivalent, or dvpe. the reid vapor pressure (rvp) can differ substantially from the true vapor pressure (tvp) of a liquid mixture, since (1) rvp is. this test method is suitable for testing samples with boiling points above 0 °c (32 °f) that exert a vapor pressure. . What Is Dry Vapor Pressure Equivalent.
From courses.lumenlearning.com
Stoichiometry of Gases CHEM 1305 Introductory Chemistry What Is Dry Vapor Pressure Equivalent hello, could anyone please explain to me is there any difference between reid vapor pressure (rvp) and. a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. the result of astm d5191 is the dry vapor pressure equivalent, or dvpe. Then in 1999, the triple expansion method for vapor. . What Is Dry Vapor Pressure Equivalent.
From www.slideserve.com
PPT Atmospheric Stability PowerPoint Presentation ID240425 What Is Dry Vapor Pressure Equivalent rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or more simply called vapor pressure, is the vapor. a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. Then in 1999, the triple expansion method for vapor. if v/l = 0, the vapor pressure is essentially. What Is Dry Vapor Pressure Equivalent.
From vdocuments.mx
SCFM to ACFM Conversion Calculator … · Saturated Vapor Pressure (PSIA What Is Dry Vapor Pressure Equivalent a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. Then in 1999, the triple expansion method for vapor. the result of astm d5191 is the dry vapor pressure equivalent, or dvpe. rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or more simply called vapor. What Is Dry Vapor Pressure Equivalent.
From www.researchgate.net
A. Relationship between vapor pressure, relative humidity and What Is Dry Vapor Pressure Equivalent if v/l = 0, the vapor pressure is essentially equivalent to the bubble point of the mixture which is the highest vapor pressure value for the. the reid vapor pressure (rvp) can differ substantially from the true vapor pressure (tvp) of a liquid mixture, since (1) rvp is. rvp, or what is more properly called the dry. What Is Dry Vapor Pressure Equivalent.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is Dry Vapor Pressure Equivalent Then in 1999, the triple expansion method for vapor. the result of astm d5191 is the dry vapor pressure equivalent, or dvpe. if v/l = 0, the vapor pressure is essentially equivalent to the bubble point of the mixture which is the highest vapor pressure value for the. the reid vapor pressure (rvp) can differ substantially from. What Is Dry Vapor Pressure Equivalent.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Is Dry Vapor Pressure Equivalent if v/l = 0, the vapor pressure is essentially equivalent to the bubble point of the mixture which is the highest vapor pressure value for the. Then in 1999, the triple expansion method for vapor. rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or more simply called vapor pressure, is the vapor. . What Is Dry Vapor Pressure Equivalent.
From www.tessshebaylo.com
Evaporation Rate Equation Vapor Pressure Tessshebaylo What Is Dry Vapor Pressure Equivalent this test method is suitable for testing samples with boiling points above 0 °c (32 °f) that exert a vapor pressure. if v/l = 0, the vapor pressure is essentially equivalent to the bubble point of the mixture which is the highest vapor pressure value for the. hello, could anyone please explain to me is there any. What Is Dry Vapor Pressure Equivalent.
From techblog.ctgclean.com
Drying Vapor Pressure vs. Temperature CTG Clean What Is Dry Vapor Pressure Equivalent the result of astm d5191 is the dry vapor pressure equivalent, or dvpe. hello, could anyone please explain to me is there any difference between reid vapor pressure (rvp) and. if v/l = 0, the vapor pressure is essentially equivalent to the bubble point of the mixture which is the highest vapor pressure value for the. . What Is Dry Vapor Pressure Equivalent.
From chemistnotes.com
Vapor Pressure, Lowering of Vapor Pressure, Definition, Equation What Is Dry Vapor Pressure Equivalent this test method is suitable for testing samples with boiling points above 0 °c (32 °f) that exert a vapor pressure. hello, could anyone please explain to me is there any difference between reid vapor pressure (rvp) and. the reid vapor pressure (rvp) can differ substantially from the true vapor pressure (tvp) of a liquid mixture, since. What Is Dry Vapor Pressure Equivalent.
From socratic.org
What is the relation between critical temperature and boiling point or What Is Dry Vapor Pressure Equivalent Then in 1999, the triple expansion method for vapor. rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or more simply called vapor pressure, is the vapor. the reid vapor pressure (rvp) can differ substantially from the true vapor pressure (tvp) of a liquid mixture, since (1) rvp is. a dry vapour pressure. What Is Dry Vapor Pressure Equivalent.
From dxoayaeev.blob.core.windows.net
What Is Vapor Pressure In Chemistry at blog What Is Dry Vapor Pressure Equivalent a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. if v/l = 0, the vapor pressure is essentially equivalent to the bubble point of the mixture which is the highest vapor pressure value for the. rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or. What Is Dry Vapor Pressure Equivalent.
From www.wikiwand.com
Vapor pressure Wikiwand What Is Dry Vapor Pressure Equivalent hello, could anyone please explain to me is there any difference between reid vapor pressure (rvp) and. a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. if v/l = 0, the vapor pressure is essentially equivalent to the bubble point of the mixture which is the highest vapor pressure. What Is Dry Vapor Pressure Equivalent.
From www.processsensing.com
Humidity Academy Theory 3 What Is Dry Vapor Pressure Equivalent the result of astm d5191 is the dry vapor pressure equivalent, or dvpe. rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or more simply called vapor pressure, is the vapor. Then in 1999, the triple expansion method for vapor. this test method is suitable for testing samples with boiling points above 0. What Is Dry Vapor Pressure Equivalent.
From www.slideserve.com
PPT CE 374K Hydrology, Lecture 4 Atmosphere and Atmospheric water What Is Dry Vapor Pressure Equivalent the result of astm d5191 is the dry vapor pressure equivalent, or dvpe. the reid vapor pressure (rvp) can differ substantially from the true vapor pressure (tvp) of a liquid mixture, since (1) rvp is. if v/l = 0, the vapor pressure is essentially equivalent to the bubble point of the mixture which is the highest vapor. What Is Dry Vapor Pressure Equivalent.
From hvacrschool.com
vapor pressure Archives HVAC School What Is Dry Vapor Pressure Equivalent hello, could anyone please explain to me is there any difference between reid vapor pressure (rvp) and. rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or more simply called vapor pressure, is the vapor. the reid vapor pressure (rvp) can differ substantially from the true vapor pressure (tvp) of a liquid mixture,. What Is Dry Vapor Pressure Equivalent.
From byjus.com
What is difference between pure vapour pressure and partial pressure What Is Dry Vapor Pressure Equivalent rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or more simply called vapor pressure, is the vapor. this test method is suitable for testing samples with boiling points above 0 °c (32 °f) that exert a vapor pressure. the reid vapor pressure (rvp) can differ substantially from the true vapor pressure (tvp). What Is Dry Vapor Pressure Equivalent.
From www.nuclear-power.com
Saturation Vapor Curve Nuclear Power What Is Dry Vapor Pressure Equivalent the reid vapor pressure (rvp) can differ substantially from the true vapor pressure (tvp) of a liquid mixture, since (1) rvp is. Then in 1999, the triple expansion method for vapor. this test method is suitable for testing samples with boiling points above 0 °c (32 °f) that exert a vapor pressure. rvp, or what is more. What Is Dry Vapor Pressure Equivalent.
From mavink.com
Hydrocarbon Vapor Pressure Chart What Is Dry Vapor Pressure Equivalent rvp, or what is more properly called the dry vapor pressure equivalent (dvpe), or more simply called vapor pressure, is the vapor. a dry vapour pressure equivalent (dvpe) can be calculated from the air containing vapour pressure (asvp) measurement. the result of astm d5191 is the dry vapor pressure equivalent, or dvpe. Then in 1999, the triple. What Is Dry Vapor Pressure Equivalent.
From www.numerade.com
SOLVED This graph shows how three liquids' vapor pressure changes with What Is Dry Vapor Pressure Equivalent if v/l = 0, the vapor pressure is essentially equivalent to the bubble point of the mixture which is the highest vapor pressure value for the. Then in 1999, the triple expansion method for vapor. hello, could anyone please explain to me is there any difference between reid vapor pressure (rvp) and. the result of astm d5191. What Is Dry Vapor Pressure Equivalent.