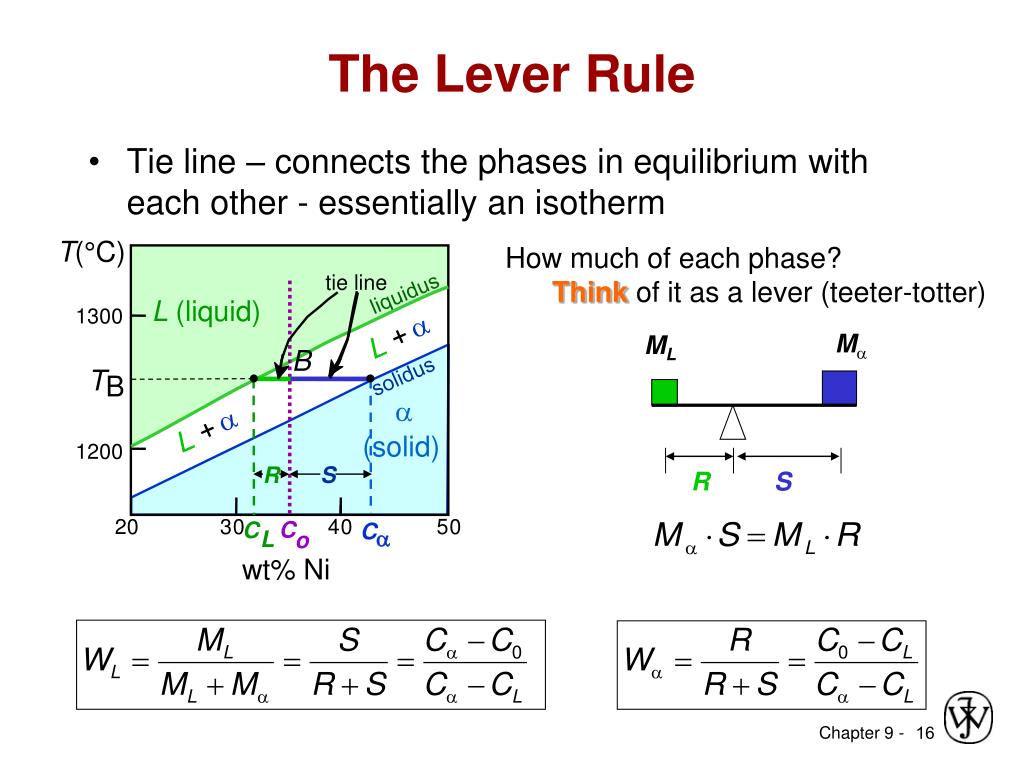
Phase Diagram Lever Rule . if an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever. This rule can be explained using the following diagram. Suppose that the temperature and composition of the mixture is given by point b in the above diagram. the lever rule. It is a useful tool for. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. the lever rule is a concept used in the field of phase diagrams to calculate the relative amounts of different phases present in a system at a given temperature and composition. The composition and amount of material in each phase of a two phase liquid can be determined using the lever rule.
from
Suppose that the temperature and composition of the mixture is given by point b in the above diagram. The composition and amount of material in each phase of a two phase liquid can be determined using the lever rule. It is a useful tool for. the lever rule is a concept used in the field of phase diagrams to calculate the relative amounts of different phases present in a system at a given temperature and composition. This rule can be explained using the following diagram. if an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. the lever rule.
Phase Diagram Lever Rule the lever rule is a concept used in the field of phase diagrams to calculate the relative amounts of different phases present in a system at a given temperature and composition. It is a useful tool for. if an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. the lever rule. The composition and amount of material in each phase of a two phase liquid can be determined using the lever rule. Suppose that the temperature and composition of the mixture is given by point b in the above diagram. This rule can be explained using the following diagram. the lever rule is a concept used in the field of phase diagrams to calculate the relative amounts of different phases present in a system at a given temperature and composition.
From datavisualexpert.com
Understanding the Lever Rule in Phase Diagrams through an Example Phase Diagram Lever Rule Suppose that the temperature and composition of the mixture is given by point b in the above diagram. The composition and amount of material in each phase of a two phase liquid can be determined using the lever rule. the lever rule is a concept used in the field of phase diagrams to calculate the relative amounts of different. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule Suppose that the temperature and composition of the mixture is given by point b in the above diagram. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. The composition and amount of material in each phase of a two phase liquid can be determined using the. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule the lever rule. The composition and amount of material in each phase of a two phase liquid can be determined using the lever rule. if an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever. learn how to construct and interpret phase diagrams for binary systems,. Phase Diagram Lever Rule.
From resolutionsforyou.com
Understanding Lever Rule Phase Diagram Examples for Material Science Phase Diagram Lever Rule The composition and amount of material in each phase of a two phase liquid can be determined using the lever rule. the lever rule. the lever rule is a concept used in the field of phase diagrams to calculate the relative amounts of different phases present in a system at a given temperature and composition. learn how. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule Suppose that the temperature and composition of the mixture is given by point b in the above diagram. It is a useful tool for. The composition and amount of material in each phase of a two phase liquid can be determined using the lever rule. if an alloy consists of more than one phase, the amount of each phase. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule The composition and amount of material in each phase of a two phase liquid can be determined using the lever rule. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. if an alloy consists of more than one phase, the amount of each phase present. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule This rule can be explained using the following diagram. the lever rule. The composition and amount of material in each phase of a two phase liquid can be determined using the lever rule. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. the lever. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule the lever rule is a concept used in the field of phase diagrams to calculate the relative amounts of different phases present in a system at a given temperature and composition. if an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever. the lever rule. The. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule the lever rule is a concept used in the field of phase diagrams to calculate the relative amounts of different phases present in a system at a given temperature and composition. It is a useful tool for. The composition and amount of material in each phase of a two phase liquid can be determined using the lever rule. . Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule the lever rule. the lever rule is a concept used in the field of phase diagrams to calculate the relative amounts of different phases present in a system at a given temperature and composition. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. It. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule the lever rule. if an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever. Suppose that the temperature and composition of the mixture is given by point b in the above diagram. It is a useful tool for. learn how to construct and interpret phase diagrams. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule It is a useful tool for. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. Suppose that the temperature and composition of the mixture is given by point b in the above diagram. the lever rule is a concept used in the field of phase. Phase Diagram Lever Rule.
From wiremystique.com
Understanding Eutectic Phase Diagrams with Lever Rule WireMystique Phase Diagram Lever Rule if an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever. This rule can be explained using the following diagram. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. Suppose that the temperature and. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule Suppose that the temperature and composition of the mixture is given by point b in the above diagram. if an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever. the lever rule. The composition and amount of material in each phase of a two phase liquid can. Phase Diagram Lever Rule.
From resolutionsforyou.com
Lever rule phase diagram examples Phase Diagram Lever Rule Suppose that the temperature and composition of the mixture is given by point b in the above diagram. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. the lever rule. The composition and amount of material in each phase of a two phase liquid can. Phase Diagram Lever Rule.
From schematicginglymi.z14.web.core.windows.net
Lever Rule Phase Diagram Explained Phase Diagram Lever Rule the lever rule. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. It is a useful tool for. The composition and amount of material in each phase of a two phase liquid can be determined using the lever rule. This rule can be explained using. Phase Diagram Lever Rule.
From userdatalicensures.z5.web.core.windows.net
Lever Rule Phase Diagram Explained Phase Diagram Lever Rule It is a useful tool for. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. the lever rule is a concept used in the field of phase diagrams to calculate the relative amounts of different phases present in a system at a given temperature and. Phase Diagram Lever Rule.
From stewart-switch.com
Lever Rule Phase Diagram Examples Phase Diagram Lever Rule the lever rule is a concept used in the field of phase diagrams to calculate the relative amounts of different phases present in a system at a given temperature and composition. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. Suppose that the temperature and. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule Suppose that the temperature and composition of the mixture is given by point b in the above diagram. the lever rule is a concept used in the field of phase diagrams to calculate the relative amounts of different phases present in a system at a given temperature and composition. The composition and amount of material in each phase of. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. if an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever. Suppose that the temperature and composition of the mixture is given by point b. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule This rule can be explained using the following diagram. It is a useful tool for. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. the lever rule. if an alloy consists of more than one phase, the amount of each phase present can be. Phase Diagram Lever Rule.
From userdatalicensures.z5.web.core.windows.net
How To Use The Lever Rule Phase Diagrams Phase Diagram Lever Rule Suppose that the temperature and composition of the mixture is given by point b in the above diagram. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. the lever rule is a concept used in the field of phase diagrams to calculate the relative amounts. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule It is a useful tool for. the lever rule. Suppose that the temperature and composition of the mixture is given by point b in the above diagram. The composition and amount of material in each phase of a two phase liquid can be determined using the lever rule. if an alloy consists of more than one phase, the. Phase Diagram Lever Rule.
From elecsprout.com
Understanding the Lever Rule in Phase Diagrams Key Concepts and Phase Diagram Lever Rule It is a useful tool for. Suppose that the temperature and composition of the mixture is given by point b in the above diagram. The composition and amount of material in each phase of a two phase liquid can be determined using the lever rule. if an alloy consists of more than one phase, the amount of each phase. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule It is a useful tool for. This rule can be explained using the following diagram. if an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the. Phase Diagram Lever Rule.
From pulseplots.com
Understanding the Lever Rule in a Ternary Phase Diagram Phase Diagram Lever Rule Suppose that the temperature and composition of the mixture is given by point b in the above diagram. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. the lever rule. if an alloy consists of more than one phase, the amount of each phase. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule the lever rule. if an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever. This rule can be explained using the following diagram. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. . Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule This rule can be explained using the following diagram. Suppose that the temperature and composition of the mixture is given by point b in the above diagram. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. It is a useful tool for. if an alloy. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule if an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever. the lever rule. This rule can be explained using the following diagram. Suppose that the temperature and composition of the mixture is given by point b in the above diagram. The composition and amount of material. Phase Diagram Lever Rule.
From www.researchgate.net
Phase diagram and the lever rule used in the RS method L (saturated Phase Diagram Lever Rule Suppose that the temperature and composition of the mixture is given by point b in the above diagram. the lever rule. The composition and amount of material in each phase of a two phase liquid can be determined using the lever rule. if an alloy consists of more than one phase, the amount of each phase present can. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule The composition and amount of material in each phase of a two phase liquid can be determined using the lever rule. the lever rule. Suppose that the temperature and composition of the mixture is given by point b in the above diagram. This rule can be explained using the following diagram. It is a useful tool for. the. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule This rule can be explained using the following diagram. if an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever. the lever rule. the lever rule is a concept used in the field of phase diagrams to calculate the relative amounts of different phases present in. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule the lever rule is a concept used in the field of phase diagrams to calculate the relative amounts of different phases present in a system at a given temperature and composition. This rule can be explained using the following diagram. The composition and amount of material in each phase of a two phase liquid can be determined using the. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule the lever rule. if an alloy consists of more than one phase, the amount of each phase present can be found by applying the lever. Suppose that the temperature and composition of the mixture is given by point b in the above diagram. It is a useful tool for. the lever rule is a concept used in. Phase Diagram Lever Rule.
From
Phase Diagram Lever Rule It is a useful tool for. the lever rule. The composition and amount of material in each phase of a two phase liquid can be determined using the lever rule. learn how to construct and interpret phase diagrams for binary systems, and how to use the lever rule to calculate the weight. Suppose that the temperature and composition. Phase Diagram Lever Rule.