Medication Labelling Requirements Uk . it sets out the legal framework for labelling and packaging as described in uk legislation. from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. the human medicines regulations 2012, cross heading: labelling labelling must contain all elements required by regulation 257 and schedule. guidance from the medicines and healthcare products regulatory agency (mhra), explains how to package. here’s what you need to know: there is a legal requirement for the following to appear on the label of any prescribed medicine: Starting from january 1, 2025, all medicine packaging in the uk must have a label that. Requirements for packaging and package leaflets relating to medicinal. Precautions relating to the use.
from www.fda.gov.ph
there is a legal requirement for the following to appear on the label of any prescribed medicine: the human medicines regulations 2012, cross heading: from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. here’s what you need to know: Requirements for packaging and package leaflets relating to medicinal. guidance from the medicines and healthcare products regulatory agency (mhra), explains how to package. it sets out the legal framework for labelling and packaging as described in uk legislation. labelling labelling must contain all elements required by regulation 257 and schedule. Precautions relating to the use. Starting from january 1, 2025, all medicine packaging in the uk must have a label that.
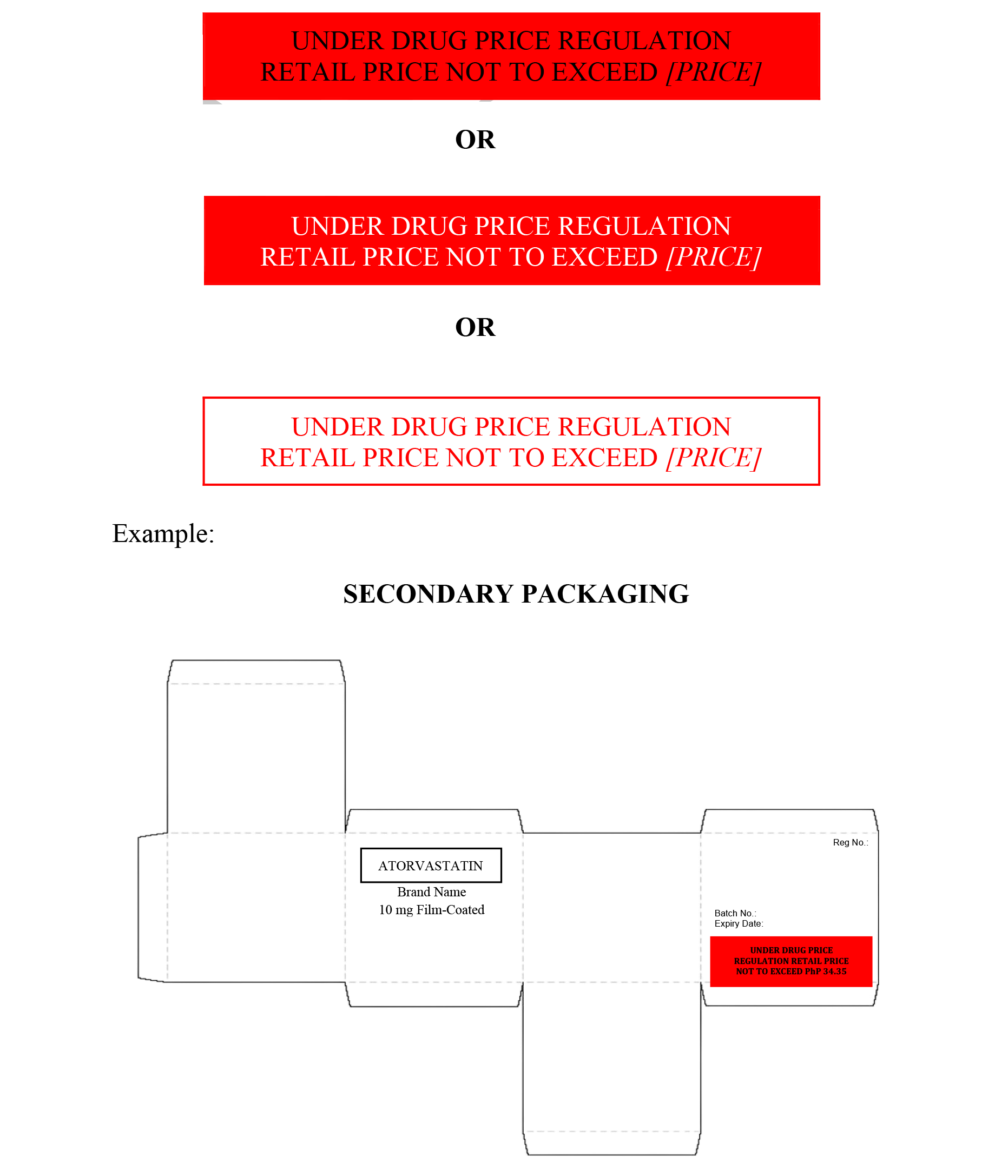
Draft for Comments Guidelines on Labeling Requirements of Drug Products under Maximum Retail
Medication Labelling Requirements Uk the human medicines regulations 2012, cross heading: labelling labelling must contain all elements required by regulation 257 and schedule. Precautions relating to the use. it sets out the legal framework for labelling and packaging as described in uk legislation. Requirements for packaging and package leaflets relating to medicinal. here’s what you need to know: from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. guidance from the medicines and healthcare products regulatory agency (mhra), explains how to package. there is a legal requirement for the following to appear on the label of any prescribed medicine: the human medicines regulations 2012, cross heading: Starting from january 1, 2025, all medicine packaging in the uk must have a label that.
From www.express-scripts.com
Understand your medication label Express Scripts® Pharmacy Medication Labelling Requirements Uk labelling labelling must contain all elements required by regulation 257 and schedule. from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. Requirements for packaging and package leaflets relating to medicinal. the human medicines regulations 2012, cross heading: here’s what you need to know: guidance from. Medication Labelling Requirements Uk.
From www.unitedadlabel.com
How To Label Medications Properly United Ad Label Medication Labelling Requirements Uk Precautions relating to the use. here’s what you need to know: there is a legal requirement for the following to appear on the label of any prescribed medicine: it sets out the legal framework for labelling and packaging as described in uk legislation. Starting from january 1, 2025, all medicine packaging in the uk must have a. Medication Labelling Requirements Uk.
From www.slideshare.net
Pharmaceutical labelling Medication Labelling Requirements Uk from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. Precautions relating to the use. guidance from the medicines and healthcare products regulatory agency (mhra), explains how to package. Requirements for packaging and package leaflets relating to medicinal. labelling labelling must contain all elements required by regulation 257. Medication Labelling Requirements Uk.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 UPDATED] Medication Labelling Requirements Uk labelling labelling must contain all elements required by regulation 257 and schedule. here’s what you need to know: it sets out the legal framework for labelling and packaging as described in uk legislation. the human medicines regulations 2012, cross heading: there is a legal requirement for the following to appear on the label of any. Medication Labelling Requirements Uk.
From healthyheels.org
Medication Label Literacy UNC Healthy Heels Medication Labelling Requirements Uk Precautions relating to the use. here’s what you need to know: the human medicines regulations 2012, cross heading: it sets out the legal framework for labelling and packaging as described in uk legislation. there is a legal requirement for the following to appear on the label of any prescribed medicine: labelling labelling must contain all. Medication Labelling Requirements Uk.
From animalia-life.club
Fda Drug Labeling Requirements Medication Labelling Requirements Uk guidance from the medicines and healthcare products regulatory agency (mhra), explains how to package. Precautions relating to the use. from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. Requirements for packaging and package leaflets relating to medicinal. labelling labelling must contain all elements required by regulation 257. Medication Labelling Requirements Uk.
From www.youtube.com
How to read a medication label YouTube Medication Labelling Requirements Uk labelling labelling must contain all elements required by regulation 257 and schedule. Precautions relating to the use. here’s what you need to know: there is a legal requirement for the following to appear on the label of any prescribed medicine: guidance from the medicines and healthcare products regulatory agency (mhra), explains how to package. Starting from. Medication Labelling Requirements Uk.
From www.slideserve.com
PPT Drug and Product Labeling PowerPoint Presentation, free download ID1818160 Medication Labelling Requirements Uk Precautions relating to the use. guidance from the medicines and healthcare products regulatory agency (mhra), explains how to package. from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. the human medicines regulations 2012, cross heading: here’s what you need to know: Requirements for packaging and package. Medication Labelling Requirements Uk.
From my.clevelandclinic.org
How To Read A Prescription Label A Guide Cleveland Clinic Medication Labelling Requirements Uk it sets out the legal framework for labelling and packaging as described in uk legislation. labelling labelling must contain all elements required by regulation 257 and schedule. from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. Precautions relating to the use. here’s what you need to. Medication Labelling Requirements Uk.
From healthyheels.org
Medication Label Literacy UNC Healthy Heels Medication Labelling Requirements Uk labelling labelling must contain all elements required by regulation 257 and schedule. it sets out the legal framework for labelling and packaging as described in uk legislation. the human medicines regulations 2012, cross heading: here’s what you need to know: there is a legal requirement for the following to appear on the label of any. Medication Labelling Requirements Uk.
From hub.arkansasbluecross.com
Deciphering Your Prescription Medication Label Blueprint Medication Labelling Requirements Uk it sets out the legal framework for labelling and packaging as described in uk legislation. guidance from the medicines and healthcare products regulatory agency (mhra), explains how to package. from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. Precautions relating to the use. here’s what you. Medication Labelling Requirements Uk.
From oliverdesign.com
Unfolding Prescription Drug Labeling Requirements Oliver Design Medication Labelling Requirements Uk labelling labelling must contain all elements required by regulation 257 and schedule. from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. guidance from the medicines and healthcare products regulatory agency (mhra), explains how to package. it sets out the legal framework for labelling and packaging as. Medication Labelling Requirements Uk.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Medication Labelling Requirements Uk there is a legal requirement for the following to appear on the label of any prescribed medicine: the human medicines regulations 2012, cross heading: guidance from the medicines and healthcare products regulatory agency (mhra), explains how to package. it sets out the legal framework for labelling and packaging as described in uk legislation. from 1. Medication Labelling Requirements Uk.
From blog.globalvision.co
Ensure Your Labels Meet all FDA Drug Labeling Requirements with Automated Quality Control Medication Labelling Requirements Uk Starting from january 1, 2025, all medicine packaging in the uk must have a label that. guidance from the medicines and healthcare products regulatory agency (mhra), explains how to package. labelling labelling must contain all elements required by regulation 257 and schedule. the human medicines regulations 2012, cross heading: there is a legal requirement for the. Medication Labelling Requirements Uk.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Medication Labelling Requirements Uk Precautions relating to the use. Requirements for packaging and package leaflets relating to medicinal. the human medicines regulations 2012, cross heading: from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. there is a legal requirement for the following to appear on the label of any prescribed medicine:. Medication Labelling Requirements Uk.
From blog.caresfield.com
Medication Labels 101 Categories, Regulations, and Best Practices Caresfield Blog Medication Labelling Requirements Uk the human medicines regulations 2012, cross heading: it sets out the legal framework for labelling and packaging as described in uk legislation. Requirements for packaging and package leaflets relating to medicinal. Starting from january 1, 2025, all medicine packaging in the uk must have a label that. labelling labelling must contain all elements required by regulation 257. Medication Labelling Requirements Uk.
From www.slideserve.com
PPT Labelling of injectable medicines, fluids and lines PowerPoint Presentation ID1544092 Medication Labelling Requirements Uk it sets out the legal framework for labelling and packaging as described in uk legislation. Precautions relating to the use. Requirements for packaging and package leaflets relating to medicinal. from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. there is a legal requirement for the following to. Medication Labelling Requirements Uk.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Medication Labelling Requirements Uk from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. Starting from january 1, 2025, all medicine packaging in the uk must have a label that. Precautions relating to the use. guidance from the medicines and healthcare products regulatory agency (mhra), explains how to package. there is a. Medication Labelling Requirements Uk.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Medication Labelling Requirements Uk there is a legal requirement for the following to appear on the label of any prescribed medicine: here’s what you need to know: Precautions relating to the use. it sets out the legal framework for labelling and packaging as described in uk legislation. Requirements for packaging and package leaflets relating to medicinal. guidance from the medicines. Medication Labelling Requirements Uk.
From brennad-images.blogspot.com
Printable Prescription Warning Labels / Ers Solutions Pharmacy Dispensing Labels Choose from Medication Labelling Requirements Uk there is a legal requirement for the following to appear on the label of any prescribed medicine: here’s what you need to know: Starting from january 1, 2025, all medicine packaging in the uk must have a label that. Requirements for packaging and package leaflets relating to medicinal. labelling labelling must contain all elements required by regulation. Medication Labelling Requirements Uk.
From grc-health.squarespace.com
Investigational Medicinal Product labelling an overview — GRCHealth Medication Labelling Requirements Uk guidance from the medicines and healthcare products regulatory agency (mhra), explains how to package. here’s what you need to know: labelling labelling must contain all elements required by regulation 257 and schedule. there is a legal requirement for the following to appear on the label of any prescribed medicine: from 1 january 2025, in order. Medication Labelling Requirements Uk.
From animalia-life.club
Fda Drug Labeling Requirements Medication Labelling Requirements Uk it sets out the legal framework for labelling and packaging as described in uk legislation. from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. Starting from january 1, 2025, all medicine packaging in the uk must have a label that. Precautions relating to the use. guidance from. Medication Labelling Requirements Uk.
From aplmed.com
4. Documenting Medications (MAR). Aplmed Academy Medication Labelling Requirements Uk it sets out the legal framework for labelling and packaging as described in uk legislation. Precautions relating to the use. there is a legal requirement for the following to appear on the label of any prescribed medicine: the human medicines regulations 2012, cross heading: Requirements for packaging and package leaflets relating to medicinal. guidance from the. Medication Labelling Requirements Uk.
From animalia-life.club
Fda Drug Labeling Requirements Medication Labelling Requirements Uk there is a legal requirement for the following to appear on the label of any prescribed medicine: Requirements for packaging and package leaflets relating to medicinal. it sets out the legal framework for labelling and packaging as described in uk legislation. the human medicines regulations 2012, cross heading: here’s what you need to know: Precautions relating. Medication Labelling Requirements Uk.
From ambitiousmares.blogspot.com
33 Pharmacy Dispensing Label Labels Design Ideas 2020 Medication Labelling Requirements Uk from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. there is a legal requirement for the following to appear on the label of any prescribed medicine: labelling labelling must contain all elements required by regulation 257 and schedule. it sets out the legal framework for labelling. Medication Labelling Requirements Uk.
From www.youtube.com
How to Read a Medication Label Nursing Skill Medication Administration Pharmacology Review Medication Labelling Requirements Uk labelling labelling must contain all elements required by regulation 257 and schedule. Starting from january 1, 2025, all medicine packaging in the uk must have a label that. there is a legal requirement for the following to appear on the label of any prescribed medicine: the human medicines regulations 2012, cross heading: guidance from the medicines. Medication Labelling Requirements Uk.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 UPDATED] Medication Labelling Requirements Uk here’s what you need to know: there is a legal requirement for the following to appear on the label of any prescribed medicine: from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. Starting from january 1, 2025, all medicine packaging in the uk must have a label. Medication Labelling Requirements Uk.
From www.fda.gov.ph
Draft for Comments Guidelines on Labeling Requirements of Drug Products under Maximum Retail Medication Labelling Requirements Uk here’s what you need to know: Starting from january 1, 2025, all medicine packaging in the uk must have a label that. Precautions relating to the use. from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. labelling labelling must contain all elements required by regulation 257 and. Medication Labelling Requirements Uk.
From giocirsmu.blob.core.windows.net
Drug Facts Box Requirements at Deleon blog Medication Labelling Requirements Uk guidance from the medicines and healthcare products regulatory agency (mhra), explains how to package. the human medicines regulations 2012, cross heading: it sets out the legal framework for labelling and packaging as described in uk legislation. from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. Starting. Medication Labelling Requirements Uk.
From etactics.com
Prescription Label Design Why It Matters and Effective Examples — Etactics Medication Labelling Requirements Uk labelling labelling must contain all elements required by regulation 257 and schedule. Starting from january 1, 2025, all medicine packaging in the uk must have a label that. Requirements for packaging and package leaflets relating to medicinal. it sets out the legal framework for labelling and packaging as described in uk legislation. here’s what you need to. Medication Labelling Requirements Uk.
From hxelpllny.blob.core.windows.net
Medical Device Labeling Uk at Tomas Prather blog Medication Labelling Requirements Uk it sets out the legal framework for labelling and packaging as described in uk legislation. Starting from january 1, 2025, all medicine packaging in the uk must have a label that. there is a legal requirement for the following to appear on the label of any prescribed medicine: from 1 january 2025, in order to enable medicines. Medication Labelling Requirements Uk.
From drugicon.cc
Drug Labelling Designs A Comparative Study Drug Icon CC 藥物圖標 Medication Labelling Requirements Uk it sets out the legal framework for labelling and packaging as described in uk legislation. there is a legal requirement for the following to appear on the label of any prescribed medicine: guidance from the medicines and healthcare products regulatory agency (mhra), explains how to package. here’s what you need to know: the human medicines. Medication Labelling Requirements Uk.
From news.uga.edu
Patientfriendly prescription labels help patients take medications Medication Labelling Requirements Uk Requirements for packaging and package leaflets relating to medicinal. here’s what you need to know: it sets out the legal framework for labelling and packaging as described in uk legislation. guidance from the medicines and healthcare products regulatory agency (mhra), explains how to package. Starting from january 1, 2025, all medicine packaging in the uk must have. Medication Labelling Requirements Uk.
From rxoutreach.org
Education Understanding Prescription Medication Labels Rx Outreach Medication Labelling Requirements Uk the human medicines regulations 2012, cross heading: here’s what you need to know: Requirements for packaging and package leaflets relating to medicinal. Precautions relating to the use. guidance from the medicines and healthcare products regulatory agency (mhra), explains how to package. Starting from january 1, 2025, all medicine packaging in the uk must have a label that.. Medication Labelling Requirements Uk.
From wholesomepharmacist.blogspot.com
The Wholesome Pharmacist How to read a label Medication Labelling Requirements Uk from 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk,. Precautions relating to the use. it sets out the legal framework for labelling and packaging as described in uk legislation. Requirements for packaging and package leaflets relating to medicinal. there is a legal requirement for the following to. Medication Labelling Requirements Uk.