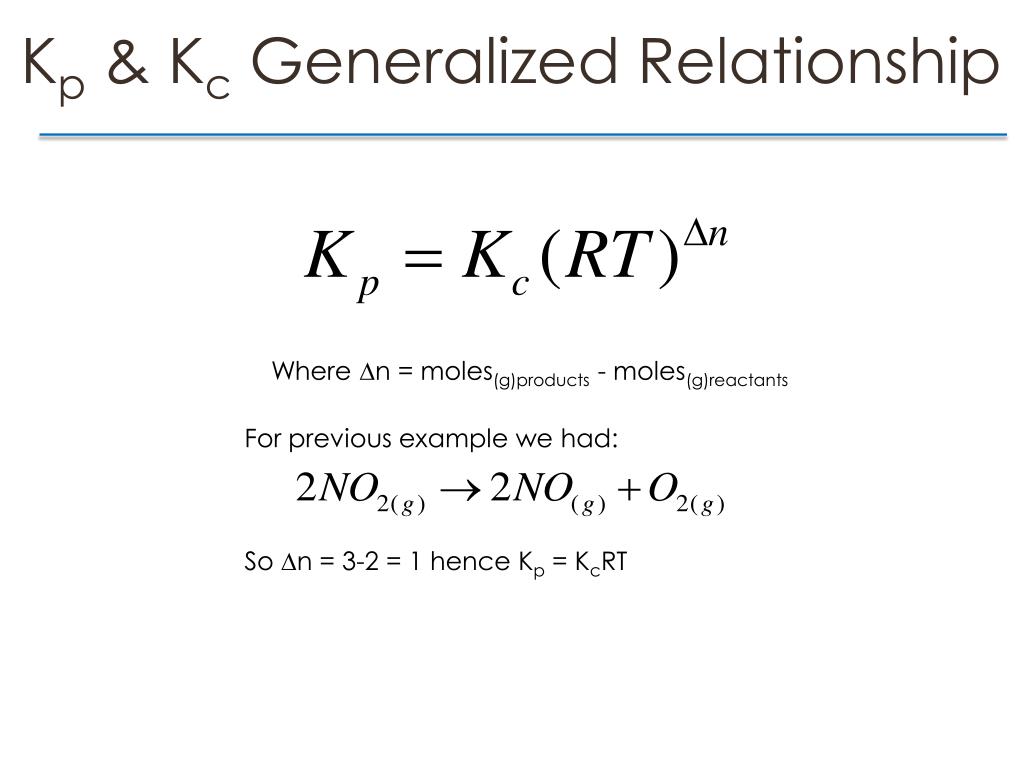
Kp Kc Reaction Examples . T he equilibrium constant for the sum of two or more reactions is the product of the equilibrium constants for each of the steps. Because \(h_2\) is a good. An example is the reaction between \(h_2\) and \(cl_2\) to produce \(hcl\), which has an equilibrium constant of \(1.6 \times 10^{33}\) at 300 k. It applies where everything in the. Equilibrium expression linking the partial pressures of reactants and products at equilibrium. However, the difference between the two constants is that \ (k_c\) is defined by molar. K p is equilibrium constant used when equilibrium concentrations are. Solids and liquids are ignored in kp equilibrium expressions. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. The kp of a reaction is constant and. The usual examples include reactions involving solids and gases, or solids and liquids. K p and k c are the equilibrium constant of an ideal gaseous mixture. For the general reaction ma + nb. This is the more straightforward case.
from www.slideserve.com
The kp of a reaction is constant and. Because \(h_2\) is a good. K p is equilibrium constant used when equilibrium concentrations are. It applies where everything in the. K p and k c are the equilibrium constant of an ideal gaseous mixture. Solids and liquids are ignored in kp equilibrium expressions. However, the difference between the two constants is that \ (k_c\) is defined by molar. Equilibrium expression linking the partial pressures of reactants and products at equilibrium. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. An example is the reaction between \(h_2\) and \(cl_2\) to produce \(hcl\), which has an equilibrium constant of \(1.6 \times 10^{33}\) at 300 k.
PPT Chapter 13 Chemical Equilibria PowerPoint Presentation, free
Kp Kc Reaction Examples Solids and liquids are ignored in kp equilibrium expressions. An example is the reaction between \(h_2\) and \(cl_2\) to produce \(hcl\), which has an equilibrium constant of \(1.6 \times 10^{33}\) at 300 k. It applies where everything in the. This is the more straightforward case. However, the difference between the two constants is that \ (k_c\) is defined by molar. K p and k c are the equilibrium constant of an ideal gaseous mixture. Equilibrium expression linking the partial pressures of reactants and products at equilibrium. K p is equilibrium constant used when equilibrium concentrations are. The usual examples include reactions involving solids and gases, or solids and liquids. Because \(h_2\) is a good. T he equilibrium constant for the sum of two or more reactions is the product of the equilibrium constants for each of the steps. For the general reaction ma + nb. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. The kp of a reaction is constant and. Solids and liquids are ignored in kp equilibrium expressions.
From www.chegg.com
Solved Write expressions for Kc, and Kp if applicable, for Kp Kc Reaction Examples T he equilibrium constant for the sum of two or more reactions is the product of the equilibrium constants for each of the steps. K p and k c are the equilibrium constant of an ideal gaseous mixture. Because \(h_2\) is a good. It applies where everything in the. An example is the reaction between \(h_2\) and \(cl_2\) to produce. Kp Kc Reaction Examples.
From haipernews.com
How To Calculate Kc Using Kp Haiper Kp Kc Reaction Examples K p and k c are the equilibrium constant of an ideal gaseous mixture. This is the more straightforward case. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. It applies where everything in the. Equilibrium expression linking the partial pressures of reactants and products at equilibrium. However, the difference between the two constants is that \. Kp Kc Reaction Examples.
From www.youtube.com
How to Write Equilibrium Constant Expression (K, Keq, Kc, Kp) Practice Kp Kc Reaction Examples K p and k c are the equilibrium constant of an ideal gaseous mixture. Solids and liquids are ignored in kp equilibrium expressions. Because \(h_2\) is a good. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. An example is the reaction between \(h_2\) and \(cl_2\) to produce \(hcl\), which has an equilibrium constant of \(1.6 \times. Kp Kc Reaction Examples.
From askfilo.com
Derive the relation between Kp and Kc for the following reactions. Filo Kp Kc Reaction Examples Because \(h_2\) is a good. The kp of a reaction is constant and. K p and k c are the equilibrium constant of an ideal gaseous mixture. It applies where everything in the. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. Solids and liquids are ignored in kp equilibrium expressions. K p is equilibrium constant used. Kp Kc Reaction Examples.
From oneclass.com
OneClass Select the reactions for which Kp is equal to Kc. Kp Kc Reaction Examples T he equilibrium constant for the sum of two or more reactions is the product of the equilibrium constants for each of the steps. K p is equilibrium constant used when equilibrium concentrations are. However, the difference between the two constants is that \ (k_c\) is defined by molar. An example is the reaction between \(h_2\) and \(cl_2\) to produce. Kp Kc Reaction Examples.
From www.numerade.com
SOLVED Question Write the equation for the conversion of Kc to Kp for Kp Kc Reaction Examples However, the difference between the two constants is that \ (k_c\) is defined by molar. T he equilibrium constant for the sum of two or more reactions is the product of the equilibrium constants for each of the steps. Equilibrium expression linking the partial pressures of reactants and products at equilibrium. Because \(h_2\) is a good. This is the more. Kp Kc Reaction Examples.
From www.slideshare.net
Chapter 15 Lecture Chemical Equilibrium Kp Kc Reaction Examples Equilibrium expression linking the partial pressures of reactants and products at equilibrium. Because \(h_2\) is a good. For the general reaction ma + nb. This is the more straightforward case. An example is the reaction between \(h_2\) and \(cl_2\) to produce \(hcl\), which has an equilibrium constant of \(1.6 \times 10^{33}\) at 300 k. \ (k_c\) and \ (k_p\) are. Kp Kc Reaction Examples.
From www.youtube.com
Converting between Kc and Kp for gas phase systems YouTube Kp Kc Reaction Examples An example is the reaction between \(h_2\) and \(cl_2\) to produce \(hcl\), which has an equilibrium constant of \(1.6 \times 10^{33}\) at 300 k. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. It applies where everything in the. However, the difference between the two constants is that \ (k_c\) is defined by molar. This is the. Kp Kc Reaction Examples.
From www.youtube.com
How to Write Equilibrium Constant Expression Kc and Kp Expression Kp Kc Reaction Examples The kp of a reaction is constant and. For the general reaction ma + nb. K p and k c are the equilibrium constant of an ideal gaseous mixture. Equilibrium expression linking the partial pressures of reactants and products at equilibrium. Solids and liquids are ignored in kp equilibrium expressions. Because \(h_2\) is a good. This is the more straightforward. Kp Kc Reaction Examples.
From www.youtube.com
Relation between Kc and Kp with one solved example YouTube Kp Kc Reaction Examples For the general reaction ma + nb. K p and k c are the equilibrium constant of an ideal gaseous mixture. The usual examples include reactions involving solids and gases, or solids and liquids. K p is equilibrium constant used when equilibrium concentrations are. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. However, the difference between. Kp Kc Reaction Examples.
From www.showme.com
Intro to Equilibrium / Kc / Kp Science, Chemistry, Chemicalreactions Kp Kc Reaction Examples Because \(h_2\) is a good. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. K p and k c are the equilibrium constant of an ideal gaseous mixture. The kp of a reaction is constant and. An example is the reaction between \(h_2\) and \(cl_2\) to produce \(hcl\), which has an equilibrium constant of \(1.6 \times 10^{33}\). Kp Kc Reaction Examples.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc YouTube Kp Kc Reaction Examples However, the difference between the two constants is that \ (k_c\) is defined by molar. K p is equilibrium constant used when equilibrium concentrations are. Solids and liquids are ignored in kp equilibrium expressions. Because \(h_2\) is a good. For the general reaction ma + nb. K p and k c are the equilibrium constant of an ideal gaseous mixture.. Kp Kc Reaction Examples.
From www.youtube.com
The equilibrium constant Kp YouTube Kp Kc Reaction Examples The kp of a reaction is constant and. K p and k c are the equilibrium constant of an ideal gaseous mixture. K p is equilibrium constant used when equilibrium concentrations are. The usual examples include reactions involving solids and gases, or solids and liquids. However, the difference between the two constants is that \ (k_c\) is defined by molar.. Kp Kc Reaction Examples.
From www.youtube.com
Write the expression for the equilibrium constant, Kc for each of the Kp Kc Reaction Examples This is the more straightforward case. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. Solids and liquids are ignored in kp equilibrium expressions. It applies where everything in the. T he equilibrium constant for the sum of two or more reactions is the product of the equilibrium constants for each of the steps. Equilibrium expression linking. Kp Kc Reaction Examples.
From www.youtube.com
Chemical Equilibrium Ice Table Equilibrium Constant Expression Kp Kc Reaction Examples For the general reaction ma + nb. It applies where everything in the. K p and k c are the equilibrium constant of an ideal gaseous mixture. Equilibrium expression linking the partial pressures of reactants and products at equilibrium. However, the difference between the two constants is that \ (k_c\) is defined by molar. An example is the reaction between. Kp Kc Reaction Examples.
From www.youtube.com
Kp and Kc YouTube Kp Kc Reaction Examples However, the difference between the two constants is that \ (k_c\) is defined by molar. The usual examples include reactions involving solids and gases, or solids and liquids. Because \(h_2\) is a good. For the general reaction ma + nb. K p is equilibrium constant used when equilibrium concentrations are. It applies where everything in the. An example is the. Kp Kc Reaction Examples.
From www.toppr.com
The relation between Kp and Kc for the reaction 2NO(g) + CI2(g) 2NOCI(g Kp Kc Reaction Examples T he equilibrium constant for the sum of two or more reactions is the product of the equilibrium constants for each of the steps. K p is equilibrium constant used when equilibrium concentrations are. For the general reaction ma + nb. Because \(h_2\) is a good. Equilibrium expression linking the partial pressures of reactants and products at equilibrium. The kp. Kp Kc Reaction Examples.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Kp Kc Reaction Examples T he equilibrium constant for the sum of two or more reactions is the product of the equilibrium constants for each of the steps. K p is equilibrium constant used when equilibrium concentrations are. Solids and liquids are ignored in kp equilibrium expressions. The usual examples include reactions involving solids and gases, or solids and liquids. \ (k_c\) and \. Kp Kc Reaction Examples.
From slideplayer.com
Chapter 14 Chemical Equilibrium ppt download Kp Kc Reaction Examples The kp of a reaction is constant and. However, the difference between the two constants is that \ (k_c\) is defined by molar. Equilibrium expression linking the partial pressures of reactants and products at equilibrium. Because \(h_2\) is a good. Solids and liquids are ignored in kp equilibrium expressions. It applies where everything in the. For the general reaction ma. Kp Kc Reaction Examples.
From www.slideserve.com
PPT Chapter 13 Chemical Equilibria PowerPoint Presentation, free Kp Kc Reaction Examples This is the more straightforward case. An example is the reaction between \(h_2\) and \(cl_2\) to produce \(hcl\), which has an equilibrium constant of \(1.6 \times 10^{33}\) at 300 k. The usual examples include reactions involving solids and gases, or solids and liquids. Equilibrium expression linking the partial pressures of reactants and products at equilibrium. T he equilibrium constant for. Kp Kc Reaction Examples.
From quizlet.com
Write the equilibrium constant expressions for Kc and Kp, if Quizlet Kp Kc Reaction Examples It applies where everything in the. For the general reaction ma + nb. Equilibrium expression linking the partial pressures of reactants and products at equilibrium. Solids and liquids are ignored in kp equilibrium expressions. T he equilibrium constant for the sum of two or more reactions is the product of the equilibrium constants for each of the steps. The kp. Kp Kc Reaction Examples.
From www.studypool.com
SOLUTION The concept of chemical equilibrium le chatelier's principle Kp Kc Reaction Examples For the general reaction ma + nb. However, the difference between the two constants is that \ (k_c\) is defined by molar. Solids and liquids are ignored in kp equilibrium expressions. It applies where everything in the. K p and k c are the equilibrium constant of an ideal gaseous mixture. K p is equilibrium constant used when equilibrium concentrations. Kp Kc Reaction Examples.
From general.chemistrysteps.com
Kp Equilibrium Constant and Partial Pressure Chemistry Steps Kp Kc Reaction Examples K p is equilibrium constant used when equilibrium concentrations are. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. This is the more straightforward case. Solids and liquids are ignored in kp equilibrium expressions. For the general reaction ma + nb. The kp of a reaction is constant and. K p and k c are the equilibrium. Kp Kc Reaction Examples.
From www.slideserve.com
PPT Chapter 15 Chemical Equilibrium PowerPoint Presentation, free Kp Kc Reaction Examples This is the more straightforward case. However, the difference between the two constants is that \ (k_c\) is defined by molar. For the general reaction ma + nb. Solids and liquids are ignored in kp equilibrium expressions. Because \(h_2\) is a good. T he equilibrium constant for the sum of two or more reactions is the product of the equilibrium. Kp Kc Reaction Examples.
From www.youtube.com
The Reaction Quotient and Example Kc and Kp problems YouTube Kp Kc Reaction Examples For the general reaction ma + nb. It applies where everything in the. K p is equilibrium constant used when equilibrium concentrations are. K p and k c are the equilibrium constant of an ideal gaseous mixture. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. An example is the reaction between \(h_2\) and \(cl_2\) to produce. Kp Kc Reaction Examples.
From www.chemicals.co.uk
Chemistry A Level Revision Equilibrium Kp Kc Reaction Examples Solids and liquids are ignored in kp equilibrium expressions. An example is the reaction between \(h_2\) and \(cl_2\) to produce \(hcl\), which has an equilibrium constant of \(1.6 \times 10^{33}\) at 300 k. Because \(h_2\) is a good. For the general reaction ma + nb. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. T he equilibrium. Kp Kc Reaction Examples.
From www.tes.com
Equilibrium Constant Expression (Kc Kp and units) ALevel Chemistry Kp Kc Reaction Examples This is the more straightforward case. Equilibrium expression linking the partial pressures of reactants and products at equilibrium. It applies where everything in the. T he equilibrium constant for the sum of two or more reactions is the product of the equilibrium constants for each of the steps. K p is equilibrium constant used when equilibrium concentrations are. An example. Kp Kc Reaction Examples.
From byjus.com
A reaction in which there is no change in volume? 1.Kp=Kc 2.Kc= 0 Which Kp Kc Reaction Examples An example is the reaction between \(h_2\) and \(cl_2\) to produce \(hcl\), which has an equilibrium constant of \(1.6 \times 10^{33}\) at 300 k. T he equilibrium constant for the sum of two or more reactions is the product of the equilibrium constants for each of the steps. For the general reaction ma + nb. Solids and liquids are ignored. Kp Kc Reaction Examples.
From www.toppr.com
Calculate Kc and Kp for the reaction at 27^o C. Kp Kc Reaction Examples The usual examples include reactions involving solids and gases, or solids and liquids. For the general reaction ma + nb. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. An example is the reaction between \(h_2\) and \(cl_2\) to produce \(hcl\), which has an equilibrium constant of \(1.6 \times 10^{33}\) at 300 k. However, the difference between. Kp Kc Reaction Examples.
From byjus.com
Kp and kc relationship below temperature 12.5k Kp Kc Reaction Examples \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. Equilibrium expression linking the partial pressures of reactants and products at equilibrium. K p and k c are the equilibrium constant of an ideal gaseous mixture. Solids and liquids are ignored in kp equilibrium expressions. T he equilibrium constant for the sum of two or more reactions is. Kp Kc Reaction Examples.
From www.slideserve.com
PPT EQUILIBRIUM CONSTANTS Kp PowerPoint Presentation, free download Kp Kc Reaction Examples Equilibrium expression linking the partial pressures of reactants and products at equilibrium. Solids and liquids are ignored in kp equilibrium expressions. K p and k c are the equilibrium constant of an ideal gaseous mixture. For the general reaction ma + nb. The usual examples include reactions involving solids and gases, or solids and liquids. K p is equilibrium constant. Kp Kc Reaction Examples.
From slideplayer.com
CHAPTER 15 Chemical Equilibrium. ppt download Kp Kc Reaction Examples Equilibrium expression linking the partial pressures of reactants and products at equilibrium. T he equilibrium constant for the sum of two or more reactions is the product of the equilibrium constants for each of the steps. An example is the reaction between \(h_2\) and \(cl_2\) to produce \(hcl\), which has an equilibrium constant of \(1.6 \times 10^{33}\) at 300 k.. Kp Kc Reaction Examples.
From chem.libretexts.org
Gas Equilibrium Constants Kc And Kp Chemistry LibreTexts Kp Kc Reaction Examples The usual examples include reactions involving solids and gases, or solids and liquids. This is the more straightforward case. Because \(h_2\) is a good. Solids and liquids are ignored in kp equilibrium expressions. K p and k c are the equilibrium constant of an ideal gaseous mixture. \ (k_c\) and \ (k_p\) are the equilibrium constants of gaseous mixtures. Equilibrium. Kp Kc Reaction Examples.
From byjus.com
correct relation of kp and kc for reaction PCl5 (g) ⇌PCl3(g)+Cl2(g) is Kp Kc Reaction Examples Equilibrium expression linking the partial pressures of reactants and products at equilibrium. This is the more straightforward case. An example is the reaction between \(h_2\) and \(cl_2\) to produce \(hcl\), which has an equilibrium constant of \(1.6 \times 10^{33}\) at 300 k. However, the difference between the two constants is that \ (k_c\) is defined by molar. For the general. Kp Kc Reaction Examples.
From www.youtube.com
Relation between Kp and Kc , Determine units of equilibrium constant Kp Kc Reaction Examples Equilibrium expression linking the partial pressures of reactants and products at equilibrium. Because \(h_2\) is a good. T he equilibrium constant for the sum of two or more reactions is the product of the equilibrium constants for each of the steps. The usual examples include reactions involving solids and gases, or solids and liquids. It applies where everything in the.. Kp Kc Reaction Examples.