Magnesium And Zinc Reaction . Magnesium atoms are losing electrons to make magnesium ions. 4.3 explain the reactivity series of metals (potassium, sodium, calcium, magnesium, aluminium, (carbon), zinc, iron, (hydrogen), copper, silver, gold) in terms of the reactivity of the metals with water and dilute acids and that these reactions show the… 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a yellow solid which. Zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Mg + zno = zn + mgo is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide. Acid + metal → salt + hydrogen. The ‘wrong’ order of magnesium and zinc might be due to oxidation on the surface of the magnesium being more extensive than for zinc. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The hydrogen ions are gaining electrons to make hydrogen molecules and so are being. The results are approximately in line with the reactivity series of the metals. Mg + zno = mgo + zn is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide.
from www.slideserve.com
The results are approximately in line with the reactivity series of the metals. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Mg + zno = mgo + zn is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide. The ‘wrong’ order of magnesium and zinc might be due to oxidation on the surface of the magnesium being more extensive than for zinc. Zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Magnesium atoms are losing electrons to make magnesium ions. The hydrogen ions are gaining electrons to make hydrogen molecules and so are being. Mg + zno = zn + mgo is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide. Acid + metal → salt + hydrogen. 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a yellow solid which.
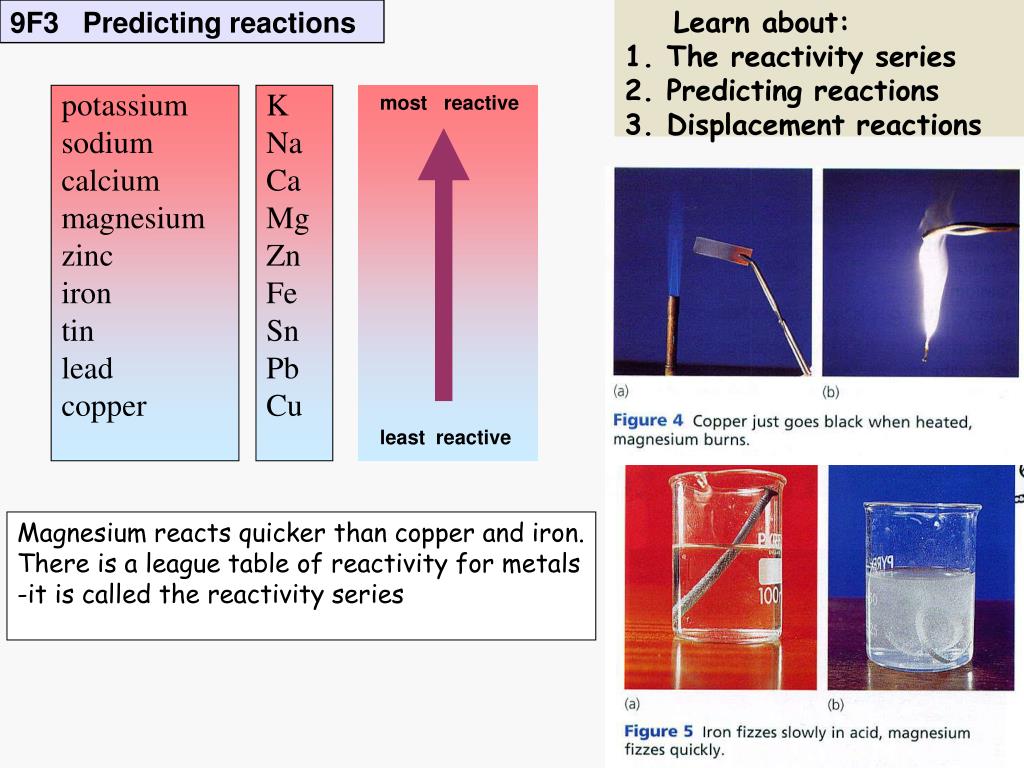
PPT Magnesium reacts quicker than copper and iron. There is a league
Magnesium And Zinc Reaction 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a yellow solid which. Acid + metal → salt + hydrogen. Mg + zno = zn + mgo is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide. Magnesium atoms are losing electrons to make magnesium ions. 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a yellow solid which. Mg + zno = mgo + zn is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide. The results are approximately in line with the reactivity series of the metals. 4.3 explain the reactivity series of metals (potassium, sodium, calcium, magnesium, aluminium, (carbon), zinc, iron, (hydrogen), copper, silver, gold) in terms of the reactivity of the metals with water and dilute acids and that these reactions show the… Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. The ‘wrong’ order of magnesium and zinc might be due to oxidation on the surface of the magnesium being more extensive than for zinc. The hydrogen ions are gaining electrons to make hydrogen molecules and so are being.
From mungfali.com
Magnesium Zinc Phase Diagram Magnesium And Zinc Reaction The hydrogen ions are gaining electrons to make hydrogen molecules and so are being. The results are approximately in line with the reactivity series of the metals. 4.3 explain the reactivity series of metals (potassium, sodium, calcium, magnesium, aluminium, (carbon), zinc, iron, (hydrogen), copper, silver, gold) in terms of the reactivity of the metals with water and dilute acids and. Magnesium And Zinc Reaction.
From www.nagwa.com
Question Video Identifying the Equation That Describes What Happens to Magnesium And Zinc Reaction Zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Acid + metal → salt + hydrogen. 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a yellow solid which. Acids will react with reactive metals,. Magnesium And Zinc Reaction.
From www.researchgate.net
Scheme 3 Synthesis of magnesium and zinc complexes 1b9b. Download Magnesium And Zinc Reaction The ‘wrong’ order of magnesium and zinc might be due to oxidation on the surface of the magnesium being more extensive than for zinc. Magnesium atoms are losing electrons to make magnesium ions. Zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Mg +. Magnesium And Zinc Reaction.
From www.youtube.com
WHEN ZINC NITRATE REACTS WITH MAGNESIUM RIBBON YouTube Magnesium And Zinc Reaction Zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Magnesium atoms are losing electrons to make magnesium ions. The hydrogen ions are gaining electrons to make hydrogen molecules and so are being. Mg + zno = mgo + zn is a single displacement (substitution). Magnesium And Zinc Reaction.
From saylordotorg.github.io
Describing Electrochemical Cells Magnesium And Zinc Reaction Acid + metal → salt + hydrogen. 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a yellow solid which. 4.3 explain the reactivity series of metals (potassium, sodium, calcium, magnesium, aluminium, (carbon), zinc, iron, (hydrogen), copper, silver, gold) in terms of the reactivity of the metals with water and dilute. Magnesium And Zinc Reaction.
From www.youtube.com
Magnesium Sulfate + Zinc YouTube Magnesium And Zinc Reaction Acid + metal → salt + hydrogen. The ‘wrong’ order of magnesium and zinc might be due to oxidation on the surface of the magnesium being more extensive than for zinc. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Mg + zno = mgo + zn is a single displacement (substitution). Magnesium And Zinc Reaction.
From recipepes.com
zinc and stainless steel reaction Magnesium And Zinc Reaction 4.3 explain the reactivity series of metals (potassium, sodium, calcium, magnesium, aluminium, (carbon), zinc, iron, (hydrogen), copper, silver, gold) in terms of the reactivity of the metals with water and dilute acids and that these reactions show the… 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a yellow solid which.. Magnesium And Zinc Reaction.
From www.youtube.com
How to Write the Net Ionic Equation for Mg + Zn(NO3)2 = Mg(NO3)2 + Zn Magnesium And Zinc Reaction The hydrogen ions are gaining electrons to make hydrogen molecules and so are being. Magnesium atoms are losing electrons to make magnesium ions. 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a yellow solid which. Mg + zno = zn + mgo is a single displacement (substitution) reaction where one. Magnesium And Zinc Reaction.
From www.numerade.com
SOLVED A 20.00 g mixture of magnesium and zinc metal reacting with Magnesium And Zinc Reaction Mg + zno = mgo + zn is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Magnesium atoms are losing electrons to make magnesium ions. 4.3 explain the reactivity series of metals (potassium, sodium,. Magnesium And Zinc Reaction.
From www.numerade.com
SOLVEDZinc and magnesium metal each react with hydrochloric acid to Magnesium And Zinc Reaction The ‘wrong’ order of magnesium and zinc might be due to oxidation on the surface of the magnesium being more extensive than for zinc. 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a yellow solid which. The hydrogen ions are gaining electrons to make hydrogen molecules and so are being.. Magnesium And Zinc Reaction.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Magnesium And Zinc Reaction The results are approximately in line with the reactivity series of the metals. Zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Mg + zno = zn + mgo is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole. Magnesium And Zinc Reaction.
From www.numerade.com
SOLVEDZinc and magnesium metal each reacts with hydrochloric acid to Magnesium And Zinc Reaction 4.3 explain the reactivity series of metals (potassium, sodium, calcium, magnesium, aluminium, (carbon), zinc, iron, (hydrogen), copper, silver, gold) in terms of the reactivity of the metals with water and dilute acids and that these reactions show the… The results are approximately in line with the reactivity series of the metals. Magnesium atoms are losing electrons to make magnesium ions.. Magnesium And Zinc Reaction.
From quizlet.com
investigate reactions between dilute hydrochloric and sulfuric acids Magnesium And Zinc Reaction 4.3 explain the reactivity series of metals (potassium, sodium, calcium, magnesium, aluminium, (carbon), zinc, iron, (hydrogen), copper, silver, gold) in terms of the reactivity of the metals with water and dilute acids and that these reactions show the… Zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one. Magnesium And Zinc Reaction.
From hxekwfnye.blob.core.windows.net
Magnesium + Zinc Sulphate Equation at Jimmy Walker blog Magnesium And Zinc Reaction Zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. The results are approximately in line with the reactivity series of the metals. Acid + metal → salt + hydrogen. Mg + zno = mgo + zn is a single displacement (substitution) reaction where one. Magnesium And Zinc Reaction.
From www.mdpi.com
Nutrients Free FullText Magnesium Biochemistry, Nutrition Magnesium And Zinc Reaction Mg + zno = mgo + zn is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide. Mg + zno = zn + mgo is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide. Acids will react with reactive metals, such as magnesium and. Magnesium And Zinc Reaction.
From www.alamy.com
Reactivity of metals. Magnesium, zinc, iron and lead in dilute Stock Magnesium And Zinc Reaction 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a yellow solid which. The results are approximately in line with the reactivity series of the metals. The ‘wrong’ order of magnesium and zinc might be due to oxidation on the surface of the magnesium being more extensive than for zinc. Acids. Magnesium And Zinc Reaction.
From autoctrls.com
The Fascinating Insight into the Magnesium Zinc Phase Diagram Magnesium And Zinc Reaction Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The ‘wrong’ order of magnesium and zinc might be due to oxidation on the surface of the magnesium being more extensive than for zinc. 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a. Magnesium And Zinc Reaction.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate Magnesium And Zinc Reaction Mg + zno = zn + mgo is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide. 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a yellow solid which. Zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction. Magnesium And Zinc Reaction.
From www.researchgate.net
Effects of magnesium and zinc on BDNF level in A549 cells. A Viability Magnesium And Zinc Reaction The results are approximately in line with the reactivity series of the metals. The ‘wrong’ order of magnesium and zinc might be due to oxidation on the surface of the magnesium being more extensive than for zinc. Magnesium atoms are losing electrons to make magnesium ions. Zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where. Magnesium And Zinc Reaction.
From www.toppr.com
21. Write chemical equations the reactions taking place when (i Magnesium And Zinc Reaction Zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Acid + metal → salt + hydrogen. Mg + zno = mgo + zn is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide. Magnesium atoms are. Magnesium And Zinc Reaction.
From www.youtube.com
Zinc Nitrate + Magnesium YouTube Magnesium And Zinc Reaction Mg + zno = mgo + zn is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide. Magnesium atoms are losing electrons to make magnesium ions. Mg + zno = zn + mgo is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide. Acids. Magnesium And Zinc Reaction.
From coggle.it
Reaction of Magnesium and Oxygen, Reaction of Zinc and Copper(II) Sulfate,… Magnesium And Zinc Reaction The hydrogen ions are gaining electrons to make hydrogen molecules and so are being. 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a yellow solid which. Magnesium atoms are losing electrons to make magnesium ions. Acids will react with reactive metals, such as magnesium and zinc, to make a salt. Magnesium And Zinc Reaction.
From mungfali.com
Magnesium Zinc Phase Diagram Magnesium And Zinc Reaction The results are approximately in line with the reactivity series of the metals. The ‘wrong’ order of magnesium and zinc might be due to oxidation on the surface of the magnesium being more extensive than for zinc. Mg + zno = mgo + zn is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of. Magnesium And Zinc Reaction.
From blog.thepipingmart.com
Zinc vs Magnesium Metals What's the Difference Magnesium And Zinc Reaction Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Magnesium atoms are losing electrons to make magnesium ions. 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a yellow solid which. The ‘wrong’ order of magnesium and zinc might be due to oxidation. Magnesium And Zinc Reaction.
From www.youtube.com
Reaction of Zinc in Magnesium and Copper Nitrate YouTube Magnesium And Zinc Reaction Zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The ‘wrong’ order of magnesium and zinc might be due to oxidation on the surface of the magnesium. Magnesium And Zinc Reaction.
From askfilo.com
We can study the reaction of metals (like magnesium, aluminium, zinc and Magnesium And Zinc Reaction The results are approximately in line with the reactivity series of the metals. The ‘wrong’ order of magnesium and zinc might be due to oxidation on the surface of the magnesium being more extensive than for zinc. Acid + metal → salt + hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and. Magnesium And Zinc Reaction.
From pubs.rsc.org
Preparation and reactions of polyfunctional magnesium and zinc Magnesium And Zinc Reaction 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a yellow solid which. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Mg + zno = zn + mgo is a single displacement (substitution) reaction where one mole of magnesium [mg] and one. Magnesium And Zinc Reaction.
From www.teachoo.com
How do Acids and Bases react with Metals? [with Examples] Teachoo Magnesium And Zinc Reaction Acid + metal → salt + hydrogen. Zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a yellow solid which. Acids will react with reactive metals,. Magnesium And Zinc Reaction.
From www.slideserve.com
PPT Magnesium reacts quicker than copper and iron. There is a league Magnesium And Zinc Reaction 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a yellow solid which. The ‘wrong’ order of magnesium and zinc might be due to oxidation on the surface of the magnesium being more extensive than for zinc. Magnesium atoms are losing electrons to make magnesium ions. Acid + metal → salt. Magnesium And Zinc Reaction.
From www.youtube.com
Redox reactions of Zinc and Magnesium YouTube Magnesium And Zinc Reaction 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature) reacts steadily when heated forming a yellow solid which. The results are approximately in line with the reactivity series of the metals. The hydrogen ions are gaining electrons to make hydrogen molecules and so are being. Magnesium atoms are losing electrons to make magnesium ions. Mg +. Magnesium And Zinc Reaction.
From www.researchgate.net
Average atomic percentatges of magnesium and zinc in the MZO buffer Magnesium And Zinc Reaction Mg + zno = mgo + zn is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide. Magnesium atoms are losing electrons to make magnesium ions. 4.3 explain the reactivity series of metals (potassium, sodium, calcium, magnesium, aluminium, (carbon), zinc, iron, (hydrogen), copper, silver, gold) in terms of the reactivity of the. Magnesium And Zinc Reaction.
From www.chegg.com
Solved REPORT SHEET LAB Chemical Reactions and Equations 10 Magnesium And Zinc Reaction Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Mg + zno = zn + mgo is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide. The ‘wrong’ order of magnesium and zinc might be due to oxidation on the surface of the magnesium. Magnesium And Zinc Reaction.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Magnesium And Zinc Reaction Magnesium atoms are losing electrons to make magnesium ions. The results are approximately in line with the reactivity series of the metals. Zn + mgso4 = mg + zn(so4) is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. 9 rows zinc (zn) reaction with oxygen (when heated and at room temperature). Magnesium And Zinc Reaction.
From mungfali.com
Magnesium Zinc Phase Diagram Magnesium And Zinc Reaction Mg + zno = zn + mgo is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide. Mg + zno = mgo + zn is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide. The hydrogen ions are gaining electrons to make hydrogen molecules. Magnesium And Zinc Reaction.
From www.researchgate.net
Scheme 3 Synthesis of magnesium and zinc complexes 1b9b. Download Magnesium And Zinc Reaction Mg + zno = zn + mgo is a single displacement (substitution) reaction where one mole of magnesium [mg] and one mole of zinc oxide. The hydrogen ions are gaining electrons to make hydrogen molecules and so are being. Magnesium atoms are losing electrons to make magnesium ions. Acids will react with reactive metals, such as magnesium and zinc, to. Magnesium And Zinc Reaction.