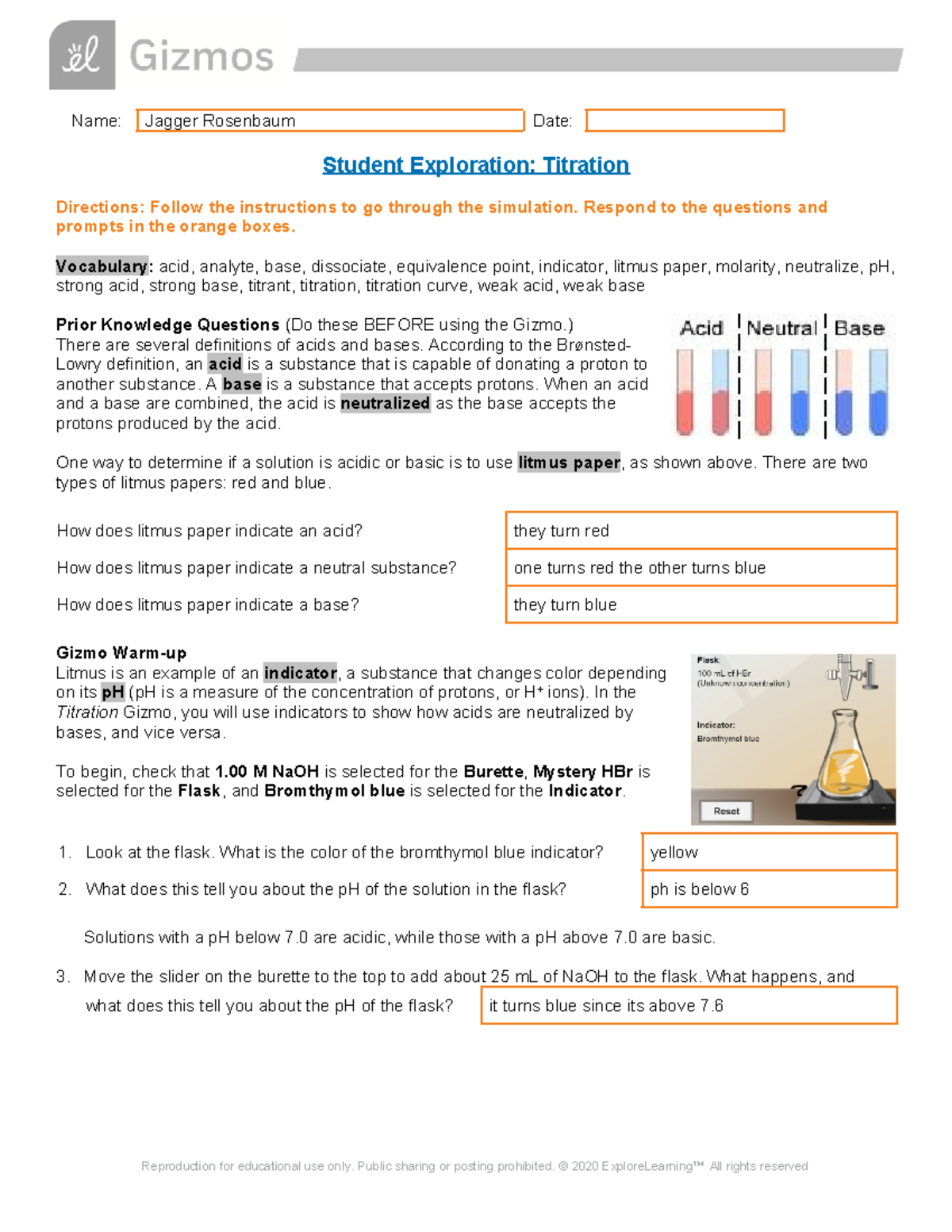
Titration Gizmo Key . To begin, check that 1 m naoh is selected for the. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1.00. To begin, check that 1.00 m naoh is. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. A titration was performed using 1.00 m naoh as the titrant and. In the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. To begin, check that 1 m naoh is selected for the burette, mystery hbr is selected for the. Use this information to calculate. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1.00 m naoh is. The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of.
from www.studocu.com
To begin, check that 1 m naoh is selected for the. A titration was performed using 1.00 m naoh as the titrant and. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1.00 m naoh is. To begin, check that 1 m naoh is selected for the burette, mystery hbr is selected for the. Use this information to calculate. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. To begin, check that 1.00 m naoh is.
Titration gizmo Name Jagger Rosenbaum Date Student Exploration
Titration Gizmo Key To begin, check that 1.00 m naoh is. A titration was performed using 1.00 m naoh as the titrant and. The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. In the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. To begin, check that 1 m naoh is selected for the. To begin, check that 1.00 m naoh is. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1.00. To begin, check that 1.00 m naoh is. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. Use this information to calculate. To begin, check that 1 m naoh is selected for the burette, mystery hbr is selected for the.
From www.studocu.com
Titration gizmo Name Jagger Rosenbaum Date Student Exploration Titration Gizmo Key To begin, check that 1 m naoh is selected for the burette, mystery hbr is selected for the. To begin, check that 1.00 m naoh is. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1.00. To begin, check that 1 m naoh is selected for. Titration Gizmo Key.
From www.studypool.com
SOLUTION Titrationse key gizmo Studypool Titration Gizmo Key Use this information to calculate. To begin, check that 1.00. To begin, check that 1.00 m naoh is. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration gizmo,. Titration Gizmo Key.
From www.studypool.com
SOLUTION Titration Student Exploration Worksheet Studypool Titration Gizmo Key A titration was performed using 1.00 m naoh as the titrant and. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is selected for. Titration Gizmo Key.
From edubirdie.com
Titration Gizmo Answer Key Virtual High School Edubirdie Titration Gizmo Key In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is selected for the. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration gizmotm, you will use indicators to show how. Titration Gizmo Key.
From tomdunnacademy.org
Unveiling the Titration Gizmo Answer Key in a PDF Format Titration Gizmo Key To begin, check that 1.00 m naoh is. Use this information to calculate. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. A titration was performed using 1.00 m naoh as the titrant and. Measure the quantity of a known solution needed to neutralize an acid or base of unknown. Titration Gizmo Key.
From edubirdie.com
Titration Gizmo Answer Key Virtual High School Edubirdie Titration Gizmo Key In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration.. Titration Gizmo Key.
From www.studocu.com
Titration and Ka gizmo Lab Titration Prelab Videos Titration in a Titration Gizmo Key To begin, check that 1 m naoh is selected for the burette, mystery hbr is selected for the. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. Measure the quantity of. Titration Gizmo Key.
From education2research.com
The Secret to Mastering Titration Unlocking the Gizmo Answer Key Titration Gizmo Key The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. A titration was performed using 1.00 m naoh as the titrant and. In the titration gizmo, you. Titration Gizmo Key.
From kguengoneclasseleven.blogspot.com
Gizmos Student Exploration Moles Answers Accel Chem Limiting Reagent Titration Gizmo Key The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. To begin, check that 1.00. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. To begin, check that 1 m naoh is selected for the burette, mystery hbr. Titration Gizmo Key.
From educacion.cc
Explore Learning Titration Gizmo Answer Key (2024). Online Education Titration Gizmo Key In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1.00 m naoh is. Use this information to calculate. The reaction of hydrochloric acid (hcl) with ammonia (nh3). Titration Gizmo Key.
From studyfinder.org
Mastering Titrations Unlocking the Student Exploration Gizmo with Titration Gizmo Key Use this information to calculate. To begin, check that 1 m naoh is selected for the. The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. In the titration. Titration Gizmo Key.
From yfxcuhpqaa.blogspot.com
Student Exploration Titration Gizmo Answer Key Gizmo Student Titration Gizmo Key The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. To begin, check that 1.00 m naoh is. Use this information to calculate. To begin, check that 1 m naoh is selected for the. A titration was performed using 1.00 m naoh as the titrant and.. Titration Gizmo Key.
From tomdunnacademy.org
Acing the Titration Gizmo Quiz Unveiling the Perfect Answers Titration Gizmo Key To begin, check that 1.00. To begin, check that 1 m naoh is selected for the. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. Use this information to calculate. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. In. Titration Gizmo Key.
From www.scribd.com
Unit 4 Activity 7 Titration Gizmo PDF Titration Chemistry Titration Gizmo Key A titration was performed using 1.00 m naoh as the titrant and. In the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1.00 m naoh is. To begin, check that 1.00 m naoh is. In the titration gizmo, you will use indicators to show how acids are. Titration Gizmo Key.
From www.scribd.com
gizmo titration worksheet Titration Chemistry Titration Gizmo Key Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. To begin, check that 1.00. To begin, check that 1 m naoh is selected for the. The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. Use this information. Titration Gizmo Key.
From www.dochub.com
Titration gizmo answer key Fill out & sign online DocHub Titration Gizmo Key To begin, check that 1.00 m naoh is. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is selected for the. To begin, check that 1.00 m naoh is. To begin, check that 1 m naoh is selected for the burette, mystery hbr. Titration Gizmo Key.
From www.studocu.com
Titration Gizmo Lab AP Chemistry Conducting a Titration Using a known Titration Gizmo Key In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. To begin, check that 1.00. To begin, check that 1.00 m naoh is. In the titration gizmo, you will use indicators to show how. Titration Gizmo Key.
From tomdunnacademy.org
Acing the Titration Gizmo Quiz Unveiling the Perfect Answers Titration Gizmo Key The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. To begin, check that 1 m naoh is selected for the burette, mystery hbr is selected for the. To begin, check that 1.00. In the titration gizmo, you will use indicators to show how acids are. Titration Gizmo Key.
From edubirdie.com
Titration Gizmo Answer Key Virtual High School Edubirdie Titration Gizmo Key In the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1.00. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration.. Titration Gizmo Key.
From www.studocu.com
Copy of Gr.11 Gizmo for online students Titration SE HNO 3 Name Titration Gizmo Key Use this information to calculate. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is selected for the burette, mystery hbr is selected for. Titration Gizmo Key.
From www.studocu.com
Titration (Gizmo) Lab 201 Titration (Gizmo) Lab Vocabulary acid Titration Gizmo Key The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is selected for the burette, mystery hbr is selected for. Titration Gizmo Key.
From qwivy.com
Exam Gizmos Student Exploration Titration Answer Key Gizmos Student Titration Gizmo Key A titration was performed using 1.00 m naoh as the titrant and. To begin, check that 1.00 m naoh is. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. The reaction. Titration Gizmo Key.
From www.studypool.com
SOLUTION Titrationse key gizmo Studypool Titration Gizmo Key In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1.00. Titration Gizmo Key.
From nonexistentibus.blogspot.com
Gizmos Moles Answer Sheet Gizmo Titration Worksheet Titration Titration Gizmo Key A titration was performed using 1.00 m naoh as the titrant and. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. In the titration gizmo, you will use indicators to show how acids. Titration Gizmo Key.
From www.scribd.com
TitrationSE Key Gizmo PDF Titration Chemistry Titration Gizmo Key In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. Use this information to calculate. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is selected for the. Measure the quantity of a known. Titration Gizmo Key.
From edubirdie.com
Titration Gizmo Answer Key Virtual High School Edubirdie Titration Gizmo Key Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. To begin, check that 1 m naoh is selected for the burette, mystery hbr is selected for the. To begin, check that 1.00 m naoh is. To begin, check that 1 m naoh is selected for the. The reaction of hydrochloric acid (hcl). Titration Gizmo Key.
From www.studocu.com
Titration Gizmo Student Exploration Titration Activity A Acids and Titration Gizmo Key To begin, check that 1.00 m naoh is. To begin, check that 1 m naoh is selected for the burette, mystery hbr is selected for the. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration gizmo, you will use indicators to show how acids are neutralized by. Titration Gizmo Key.
From www.studocu.com
Titration SE Key Gizmo This is about tritation 2019 Titration Titration Gizmo Key The reaction of hydrochloric acid (hcl) with ammonia (nh3) is described by the equation:hcl + nh3 → nh4cla student is titrating 50 ml of. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and. Titration Gizmo Key.
From edubirdie.com
Titration Gizmo Answer Key Virtual High School Edubirdie Titration Gizmo Key To begin, check that 1 m naoh is selected for the. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1.00 m naoh is. To begin, check that 1 m naoh is selected for the burette, mystery hbr is selected for the. Use this information to. Titration Gizmo Key.
From purchasingyamaha88keys.blogspot.com
Calorimetry Lab Gizmo Answers Activity C Flavio Tonon It Follow the Titration Gizmo Key To begin, check that 1.00. Use this information to calculate. To begin, check that 1 m naoh is selected for the burette, mystery hbr is selected for the. In the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is selected for the. To begin,. Titration Gizmo Key.
From issuu.com
Titration gizmo answer key teacher guide by Ebook Manual PDF Issuu Titration Gizmo Key To begin, check that 1.00 m naoh is. To begin, check that 1.00 m naoh is. In the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. Measure the quantity of a known solution needed to neutralize an acid or base of unknown concentration. The reaction of hydrochloric acid (hcl) with ammonia. Titration Gizmo Key.
From edubirdie.com
Titration Gizmo Answer Key Virtual High School Edubirdie Titration Gizmo Key In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. Measure the quantity. Titration Gizmo Key.
From edubirdie.com
Titration Gizmo Answer Key Virtual High School Edubirdie Titration Gizmo Key In the titration gizmotm, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. Measure the quantity. Titration Gizmo Key.
From edubirdie.com
Titration Gizmo Answer Key Virtual High School Edubirdie Titration Gizmo Key To begin, check that 1.00 m naoh is. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is selected for the burette, mystery hbr is selected for the. In the titration gizmotm, you will use indicators to show how acids are neutralized by. Titration Gizmo Key.
From www.studypool.com
SOLUTION Titrationse key gizmo Studypool Titration Gizmo Key To begin, check that 1.00 m naoh is. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. To begin, check that 1 m naoh is selected for the. In the titration gizmo, you will use indicators to show how acids are neutralized by bases, and vice versa. In the titration. Titration Gizmo Key.