How Do You Calculate Mass Ratio . Start by identifying the molar ratios between the reactants and products from the balanced chemical equations. Molar mass is a useful chemical ratio between. For each compound, find the grams of copper that combine with \(1.00 \: Before applying stoichiometric factors to chemical equations, you need to understand molar mass. Use the molar ratios to calculate the amounts of reactants or. \text{g}\) of chlorine by dividing the mass of copper by the. Compound a = 4.08 g cu and 2.28 g cl. Chemists use relative atomic masses and relative formula masses to carry out mole. Firstly, the formula mass of aluminium oxide can be calculated using the formula and masses found in the data book. This formula triangle can be used to explain the relationship. Compound b = 7.53 g cu and 8.40 g cl. List the known quantities and plan the problem. Given the molecular formula of any compound as well as a periodic table of the elements, calculate the compound's mass ratio, also called the percent composition. Apply the law of multiple. The mass ratio in chemistry can be calculated by dividing the mass of one substance by the mass of another substance.
from www.showme.com
The mass ratio in chemistry can be calculated by dividing the mass of one substance by the mass of another substance. Start by identifying the molar ratios between the reactants and products from the balanced chemical equations. Firstly, the formula mass of aluminium oxide can be calculated using the formula and masses found in the data book. This formula triangle can be used to explain the relationship. List the known quantities and plan the problem. Chemists use relative atomic masses and relative formula masses to carry out mole. Compound b = 7.53 g cu and 8.40 g cl. Apply the law of multiple. Given the molecular formula of any compound as well as a periodic table of the elements, calculate the compound's mass ratio, also called the percent composition. Compound a = 4.08 g cu and 2.28 g cl.
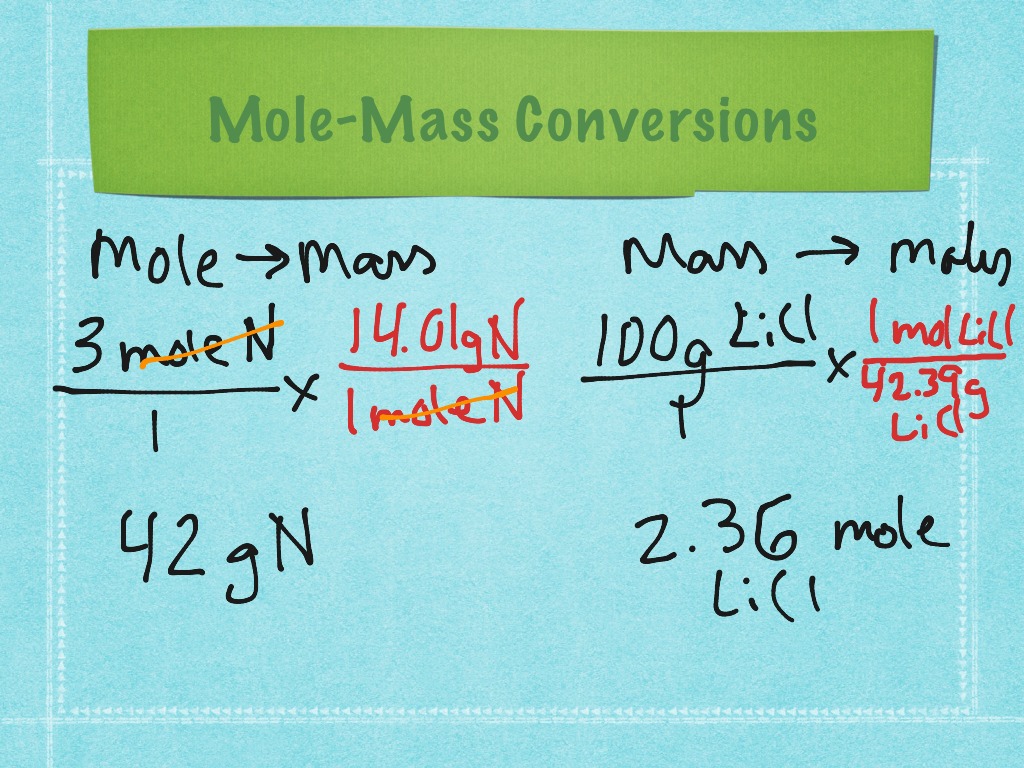
Mole and mass conversions Science, Chemistry, Stoichiometry ShowMe
How Do You Calculate Mass Ratio This formula triangle can be used to explain the relationship. \text{g}\) of chlorine by dividing the mass of copper by the. This formula triangle can be used to explain the relationship. The mass ratio in chemistry can be calculated by dividing the mass of one substance by the mass of another substance. Firstly, the formula mass of aluminium oxide can be calculated using the formula and masses found in the data book. Apply the law of multiple. Use the molar ratios to calculate the amounts of reactants or. Compound b = 7.53 g cu and 8.40 g cl. For each compound, find the grams of copper that combine with \(1.00 \: Chemists use relative atomic masses and relative formula masses to carry out mole. List the known quantities and plan the problem. Start by identifying the molar ratios between the reactants and products from the balanced chemical equations. Molar mass is a useful chemical ratio between. Given the molecular formula of any compound as well as a periodic table of the elements, calculate the compound's mass ratio, also called the percent composition. Before applying stoichiometric factors to chemical equations, you need to understand molar mass. Compound a = 4.08 g cu and 2.28 g cl.
From www.nagwa.com
Question Video Calculating the ChargetoMass Ratio of a Particle How Do You Calculate Mass Ratio Use the molar ratios to calculate the amounts of reactants or. Molar mass is a useful chemical ratio between. For each compound, find the grams of copper that combine with \(1.00 \: \text{g}\) of chlorine by dividing the mass of copper by the. This formula triangle can be used to explain the relationship. Compound a = 4.08 g cu and. How Do You Calculate Mass Ratio.
From www.sliderbase.com
The Mole Presentation Chemistry How Do You Calculate Mass Ratio Firstly, the formula mass of aluminium oxide can be calculated using the formula and masses found in the data book. Apply the law of multiple. Compound a = 4.08 g cu and 2.28 g cl. Use the molar ratios to calculate the amounts of reactants or. Chemists use relative atomic masses and relative formula masses to carry out mole. \text{g}\). How Do You Calculate Mass Ratio.
From studylib.net
EXAMPLE 2.1 Mass Ratios continued How Do You Calculate Mass Ratio Firstly, the formula mass of aluminium oxide can be calculated using the formula and masses found in the data book. Start by identifying the molar ratios between the reactants and products from the balanced chemical equations. The mass ratio in chemistry can be calculated by dividing the mass of one substance by the mass of another substance. Compound a =. How Do You Calculate Mass Ratio.
From general.chemistrysteps.com
Stoichiometry of Chemical Reactions Chemistry Steps How Do You Calculate Mass Ratio List the known quantities and plan the problem. Compound a = 4.08 g cu and 2.28 g cl. Use the molar ratios to calculate the amounts of reactants or. Given the molecular formula of any compound as well as a periodic table of the elements, calculate the compound's mass ratio, also called the percent composition. Before applying stoichiometric factors to. How Do You Calculate Mass Ratio.
From www.nagwa.com
Question Video Using the ChargetoMass Ratio to Find the Mass of a How Do You Calculate Mass Ratio Given the molecular formula of any compound as well as a periodic table of the elements, calculate the compound's mass ratio, also called the percent composition. Before applying stoichiometric factors to chemical equations, you need to understand molar mass. Start by identifying the molar ratios between the reactants and products from the balanced chemical equations. Use the molar ratios to. How Do You Calculate Mass Ratio.
From www.tessshebaylo.com
Mole Ratios How Can The Coefficients In A Chemical Equation Be How Do You Calculate Mass Ratio Start by identifying the molar ratios between the reactants and products from the balanced chemical equations. This formula triangle can be used to explain the relationship. Chemists use relative atomic masses and relative formula masses to carry out mole. Apply the law of multiple. Compound a = 4.08 g cu and 2.28 g cl. Molar mass is a useful chemical. How Do You Calculate Mass Ratio.
From www.youtube.com
Structure of Atom Class 11 Chemistry Charge to Mass Ratio of How Do You Calculate Mass Ratio For each compound, find the grams of copper that combine with \(1.00 \: \text{g}\) of chlorine by dividing the mass of copper by the. Start by identifying the molar ratios between the reactants and products from the balanced chemical equations. Apply the law of multiple. Firstly, the formula mass of aluminium oxide can be calculated using the formula and masses. How Do You Calculate Mass Ratio.
From www.youtube.com
How to calculate average atomic mass YouTube How Do You Calculate Mass Ratio Given the molecular formula of any compound as well as a periodic table of the elements, calculate the compound's mass ratio, also called the percent composition. For each compound, find the grams of copper that combine with \(1.00 \: Firstly, the formula mass of aluminium oxide can be calculated using the formula and masses found in the data book. Compound. How Do You Calculate Mass Ratio.
From www.youtube.com
Using Mass Ratios to Solve Chemistry Problems YouTube How Do You Calculate Mass Ratio Chemists use relative atomic masses and relative formula masses to carry out mole. Start by identifying the molar ratios between the reactants and products from the balanced chemical equations. This formula triangle can be used to explain the relationship. Given the molecular formula of any compound as well as a periodic table of the elements, calculate the compound's mass ratio,. How Do You Calculate Mass Ratio.
From www.youtube.com
Unit 4 Using mass ratios to predict formulas YouTube How Do You Calculate Mass Ratio \text{g}\) of chlorine by dividing the mass of copper by the. Start by identifying the molar ratios between the reactants and products from the balanced chemical equations. Molar mass is a useful chemical ratio between. Chemists use relative atomic masses and relative formula masses to carry out mole. For each compound, find the grams of copper that combine with \(1.00. How Do You Calculate Mass Ratio.
From www.slideserve.com
PPT Stoichiometry Calculations with Chemical Formulas and Equation How Do You Calculate Mass Ratio Firstly, the formula mass of aluminium oxide can be calculated using the formula and masses found in the data book. For each compound, find the grams of copper that combine with \(1.00 \: The mass ratio in chemistry can be calculated by dividing the mass of one substance by the mass of another substance. Compound b = 7.53 g cu. How Do You Calculate Mass Ratio.
From www.youtube.com
Percent Composition By Mass YouTube How Do You Calculate Mass Ratio Apply the law of multiple. Compound a = 4.08 g cu and 2.28 g cl. Molar mass is a useful chemical ratio between. Before applying stoichiometric factors to chemical equations, you need to understand molar mass. The mass ratio in chemistry can be calculated by dividing the mass of one substance by the mass of another substance. For each compound,. How Do You Calculate Mass Ratio.
From www.tessshebaylo.com
Mole Ratios How Can The Coefficients In A Chemical Equation Be How Do You Calculate Mass Ratio For each compound, find the grams of copper that combine with \(1.00 \: List the known quantities and plan the problem. Compound b = 7.53 g cu and 8.40 g cl. Firstly, the formula mass of aluminium oxide can be calculated using the formula and masses found in the data book. \text{g}\) of chlorine by dividing the mass of copper. How Do You Calculate Mass Ratio.
From physicscalculatorpro.com
Online Volume to Mass Calculator How do you calculate volume using How Do You Calculate Mass Ratio Compound b = 7.53 g cu and 8.40 g cl. Apply the law of multiple. The mass ratio in chemistry can be calculated by dividing the mass of one substance by the mass of another substance. Start by identifying the molar ratios between the reactants and products from the balanced chemical equations. List the known quantities and plan the problem.. How Do You Calculate Mass Ratio.
From www.youtube.com
Calculating relative atomic mass YouTube How Do You Calculate Mass Ratio List the known quantities and plan the problem. The mass ratio in chemistry can be calculated by dividing the mass of one substance by the mass of another substance. This formula triangle can be used to explain the relationship. Use the molar ratios to calculate the amounts of reactants or. Firstly, the formula mass of aluminium oxide can be calculated. How Do You Calculate Mass Ratio.
From www.youtube.com
Charge to mass ratio of electron proof (equation derivation used in j j How Do You Calculate Mass Ratio Compound b = 7.53 g cu and 8.40 g cl. Use the molar ratios to calculate the amounts of reactants or. Apply the law of multiple. Before applying stoichiometric factors to chemical equations, you need to understand molar mass. The mass ratio in chemistry can be calculated by dividing the mass of one substance by the mass of another substance.. How Do You Calculate Mass Ratio.
From www.youtube.com
Calculations of reacting masses from chemical equations. YouTube How Do You Calculate Mass Ratio For each compound, find the grams of copper that combine with \(1.00 \: This formula triangle can be used to explain the relationship. Use the molar ratios to calculate the amounts of reactants or. Compound a = 4.08 g cu and 2.28 g cl. Compound b = 7.53 g cu and 8.40 g cl. List the known quantities and plan. How Do You Calculate Mass Ratio.
From www.wikihow.com
3 Clear and Easy Ways to Calculate Atomic Mass wikiHow How Do You Calculate Mass Ratio Firstly, the formula mass of aluminium oxide can be calculated using the formula and masses found in the data book. Use the molar ratios to calculate the amounts of reactants or. This formula triangle can be used to explain the relationship. Molar mass is a useful chemical ratio between. Given the molecular formula of any compound as well as a. How Do You Calculate Mass Ratio.
From www.toppr.com
The charge to mass ratio of electron is found to be How Do You Calculate Mass Ratio List the known quantities and plan the problem. For each compound, find the grams of copper that combine with \(1.00 \: Firstly, the formula mass of aluminium oxide can be calculated using the formula and masses found in the data book. Start by identifying the molar ratios between the reactants and products from the balanced chemical equations. Apply the law. How Do You Calculate Mass Ratio.
From www.vrogue.co
How To Calculate Mass Ratio Sciencing vrogue.co How Do You Calculate Mass Ratio Chemists use relative atomic masses and relative formula masses to carry out mole. For each compound, find the grams of copper that combine with \(1.00 \: Before applying stoichiometric factors to chemical equations, you need to understand molar mass. \text{g}\) of chlorine by dividing the mass of copper by the. Use the molar ratios to calculate the amounts of reactants. How Do You Calculate Mass Ratio.
From www.wikihow.com
How to Calculate Mass Percent 13 Steps (with Pictures) wikiHow How Do You Calculate Mass Ratio Compound b = 7.53 g cu and 8.40 g cl. Compound a = 4.08 g cu and 2.28 g cl. This formula triangle can be used to explain the relationship. Molar mass is a useful chemical ratio between. The mass ratio in chemistry can be calculated by dividing the mass of one substance by the mass of another substance. Before. How Do You Calculate Mass Ratio.
From www.youtube.com
Calculate the mass percentage of hydrogen and oxygen in water. YouTube How Do You Calculate Mass Ratio For each compound, find the grams of copper that combine with \(1.00 \: Before applying stoichiometric factors to chemical equations, you need to understand molar mass. List the known quantities and plan the problem. Start by identifying the molar ratios between the reactants and products from the balanced chemical equations. Given the molecular formula of any compound as well as. How Do You Calculate Mass Ratio.
From www.showme.com
Mole and mass conversions Science, Chemistry, Stoichiometry ShowMe How Do You Calculate Mass Ratio This formula triangle can be used to explain the relationship. For each compound, find the grams of copper that combine with \(1.00 \: Apply the law of multiple. Use the molar ratios to calculate the amounts of reactants or. Molar mass is a useful chemical ratio between. Chemists use relative atomic masses and relative formula masses to carry out mole.. How Do You Calculate Mass Ratio.
From masterconceptsinchemistry.com
How to calculate atomic mass of an isotope given the isotopic mass and How Do You Calculate Mass Ratio Start by identifying the molar ratios between the reactants and products from the balanced chemical equations. Apply the law of multiple. Before applying stoichiometric factors to chemical equations, you need to understand molar mass. Given the molecular formula of any compound as well as a periodic table of the elements, calculate the compound's mass ratio, also called the percent composition.. How Do You Calculate Mass Ratio.
From quizexurbanite.z21.web.core.windows.net
How To Calculate The Molecular Mass How Do You Calculate Mass Ratio Firstly, the formula mass of aluminium oxide can be calculated using the formula and masses found in the data book. For each compound, find the grams of copper that combine with \(1.00 \: Use the molar ratios to calculate the amounts of reactants or. Given the molecular formula of any compound as well as a periodic table of the elements,. How Do You Calculate Mass Ratio.
From www.youtube.com
Mass Ratio YouTube How Do You Calculate Mass Ratio List the known quantities and plan the problem. The mass ratio in chemistry can be calculated by dividing the mass of one substance by the mass of another substance. Given the molecular formula of any compound as well as a periodic table of the elements, calculate the compound's mass ratio, also called the percent composition. Molar mass is a useful. How Do You Calculate Mass Ratio.
From www.pinterest.com
A mole conversion is when the mole is able the measure both mass and How Do You Calculate Mass Ratio List the known quantities and plan the problem. Chemists use relative atomic masses and relative formula masses to carry out mole. Firstly, the formula mass of aluminium oxide can be calculated using the formula and masses found in the data book. Given the molecular formula of any compound as well as a periodic table of the elements, calculate the compound's. How Do You Calculate Mass Ratio.
From www.vrogue.co
How To Calculate Mass Ratio Sciencing vrogue.co How Do You Calculate Mass Ratio This formula triangle can be used to explain the relationship. Compound b = 7.53 g cu and 8.40 g cl. Start by identifying the molar ratios between the reactants and products from the balanced chemical equations. Molar mass is a useful chemical ratio between. For each compound, find the grams of copper that combine with \(1.00 \: List the known. How Do You Calculate Mass Ratio.
From www.askiitians.com
IIT JEE Measurement of Mass and Weight JEE General Physics Study Material How Do You Calculate Mass Ratio List the known quantities and plan the problem. The mass ratio in chemistry can be calculated by dividing the mass of one substance by the mass of another substance. Compound b = 7.53 g cu and 8.40 g cl. Given the molecular formula of any compound as well as a periodic table of the elements, calculate the compound's mass ratio,. How Do You Calculate Mass Ratio.
From byjus.com
how to calculate ratios of isotopes for average atomic mass How Do You Calculate Mass Ratio For each compound, find the grams of copper that combine with \(1.00 \: Molar mass is a useful chemical ratio between. Compound a = 4.08 g cu and 2.28 g cl. Use the molar ratios to calculate the amounts of reactants or. This formula triangle can be used to explain the relationship. Before applying stoichiometric factors to chemical equations, you. How Do You Calculate Mass Ratio.
From www.youtube.com
mass to mass ratio YouTube How Do You Calculate Mass Ratio Compound a = 4.08 g cu and 2.28 g cl. Given the molecular formula of any compound as well as a periodic table of the elements, calculate the compound's mass ratio, also called the percent composition. Use the molar ratios to calculate the amounts of reactants or. Before applying stoichiometric factors to chemical equations, you need to understand molar mass.. How Do You Calculate Mass Ratio.
From www.nagwa.com
Question Video Calculating the ChargetoMass Ratio of a Neutron Nagwa How Do You Calculate Mass Ratio Compound b = 7.53 g cu and 8.40 g cl. Molar mass is a useful chemical ratio between. For each compound, find the grams of copper that combine with \(1.00 \: Start by identifying the molar ratios between the reactants and products from the balanced chemical equations. Compound a = 4.08 g cu and 2.28 g cl. Given the molecular. How Do You Calculate Mass Ratio.
From design.udlvirtual.edu.pe
How Do You Calculate The Surface Area To Volume Ratio Of A Cube How Do You Calculate Mass Ratio Before applying stoichiometric factors to chemical equations, you need to understand molar mass. Firstly, the formula mass of aluminium oxide can be calculated using the formula and masses found in the data book. Use the molar ratios to calculate the amounts of reactants or. \text{g}\) of chlorine by dividing the mass of copper by the. Given the molecular formula of. How Do You Calculate Mass Ratio.
From www.nagwa.com
Lesson ChargetoMass Ratio Nagwa How Do You Calculate Mass Ratio Compound a = 4.08 g cu and 2.28 g cl. Apply the law of multiple. Chemists use relative atomic masses and relative formula masses to carry out mole. Compound b = 7.53 g cu and 8.40 g cl. \text{g}\) of chlorine by dividing the mass of copper by the. Start by identifying the molar ratios between the reactants and products. How Do You Calculate Mass Ratio.
From www.youtube.com
In the chemical analysis of a rock the mass ratio of two radioactive How Do You Calculate Mass Ratio Compound a = 4.08 g cu and 2.28 g cl. Apply the law of multiple. Molar mass is a useful chemical ratio between. Firstly, the formula mass of aluminium oxide can be calculated using the formula and masses found in the data book. List the known quantities and plan the problem. Before applying stoichiometric factors to chemical equations, you need. How Do You Calculate Mass Ratio.