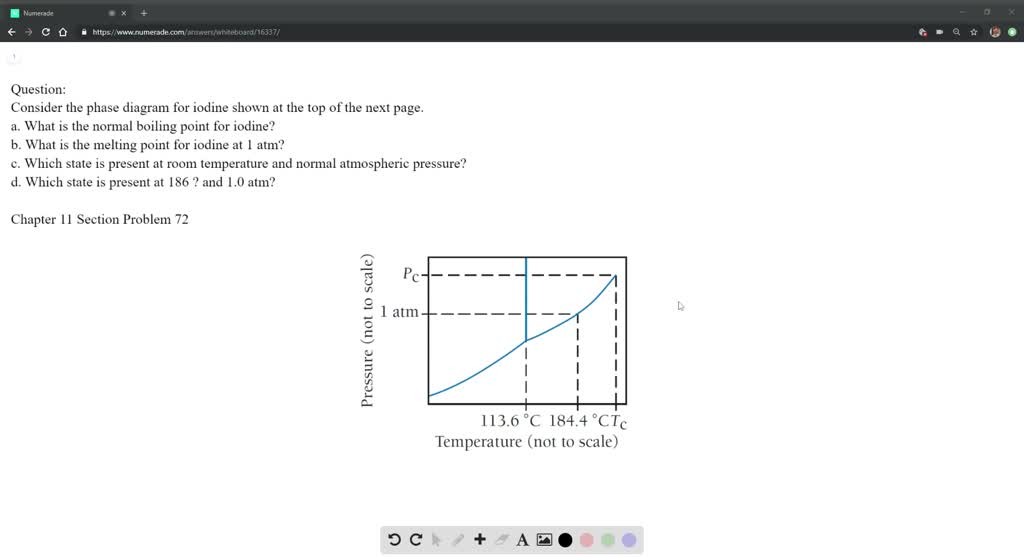
Phase Diagram Iodine . The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\. Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior. This article from uwaterloo has a phase diagram for iodine, and explains some key points. Temperature (k) a b c reference comment; Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify the normal melting and boiling points,. The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure and temperature. Coefficents calculated by nist from author's. In this paper, we present a demonstration for sublimation and we investigated the.
from solvedlib.com
Temperature (k) a b c reference comment; Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior. Coefficents calculated by nist from author's. The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\. The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure and temperature. Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify the normal melting and boiling points,. In this paper, we present a demonstration for sublimation and we investigated the. This article from uwaterloo has a phase diagram for iodine, and explains some key points.
Consider the phase diagram for iodine shown at the to… SolvedLib
Phase Diagram Iodine Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify the normal melting and boiling points,. Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior. Coefficents calculated by nist from author's. The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure and temperature. This article from uwaterloo has a phase diagram for iodine, and explains some key points. Temperature (k) a b c reference comment; The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\. Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify the normal melting and boiling points,. In this paper, we present a demonstration for sublimation and we investigated the.
From www.researchgate.net
Ternary phase diagram of iodine distribution Download Scientific Diagram Phase Diagram Iodine Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify the normal melting and boiling points,. Temperature (k) a b c reference comment; Coefficents calculated by nist from author's. In this paper, we present a demonstration for sublimation and we investigated the. This article from uwaterloo has a phase diagram for. Phase Diagram Iodine.
From www.chegg.com
Solved Consider the phase diagram for iodine shown here. a. Phase Diagram Iodine In this paper, we present a demonstration for sublimation and we investigated the. The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\. This article from uwaterloo has a phase diagram for iodine, and explains some key points. Temperature (k) a b c reference comment; Coefficents calculated by nist from author's. The phase diagram represents the phase that. Phase Diagram Iodine.
From wiringall.com
Iodine Electron Dot Diagram Phase Diagram Iodine Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify the normal melting and boiling points,. In this paper, we present a demonstration for sublimation and we investigated the. This article from uwaterloo has a phase diagram for iodine, and explains some key points. Solid carbon dioxide (dry ice), with its. Phase Diagram Iodine.
From www.researchgate.net
5days timeseries of several aqueous phase iodine species and iodine Phase Diagram Iodine This article from uwaterloo has a phase diagram for iodine, and explains some key points. Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior. In this paper, we present a demonstration for sublimation and we investigated the. Coefficents calculated by nist from author's. The. Phase Diagram Iodine.
From www.slideserve.com
PPT General Chemistry PowerPoint Presentation, free download ID9340448 Phase Diagram Iodine Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior. The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\. Coefficents calculated by nist from author's. This article from uwaterloo has a phase diagram for iodine, and explains some key points. Given a. Phase Diagram Iodine.
From www.sciencephoto.com
Iodine, atomic structure Stock Image C018/3734 Science Photo Library Phase Diagram Iodine In this paper, we present a demonstration for sublimation and we investigated the. The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\. Coefficents calculated by nist from author's. Temperature (k) a b c reference comment; The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure and temperature.. Phase Diagram Iodine.
From solvedlib.com
Examine the phase diagram for iodine shown in Figure … SolvedLib Phase Diagram Iodine The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\. This article from uwaterloo has a phase diagram for iodine, and explains some key points. Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior. In this paper, we present a demonstration for. Phase Diagram Iodine.
From chemistry.stackexchange.com
intermolecular forces Sublimation of Iodine Chemistry Stack Exchange Phase Diagram Iodine Temperature (k) a b c reference comment; The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure and temperature. In this paper, we present a demonstration for sublimation and we investigated the. Coefficents calculated by nist from author's. This article from uwaterloo has a phase diagram for iodine, and explains. Phase Diagram Iodine.
From diagramlibrarygermins.z19.web.core.windows.net
Iodine Phase Diagram Phase Diagram Iodine Temperature (k) a b c reference comment; Coefficents calculated by nist from author's. In this paper, we present a demonstration for sublimation and we investigated the. The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\. The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure and temperature.. Phase Diagram Iodine.
From www.tau.ac.il
Chemical Physics Details Phase Diagram Iodine The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\. The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure and temperature. Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior. Coefficents. Phase Diagram Iodine.
From www.sciencephoto.com
Iodine, atomic structure Stock Image C023/2559 Science Photo Library Phase Diagram Iodine This article from uwaterloo has a phase diagram for iodine, and explains some key points. The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure and temperature. Temperature (k) a b c reference comment; Coefficents calculated by nist from author's. Given a phase diagram of a pure substance, label all. Phase Diagram Iodine.
From wiringall.com
Iodine Electron Dot Diagram Phase Diagram Iodine Temperature (k) a b c reference comment; Coefficents calculated by nist from author's. The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure and temperature. Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior.. Phase Diagram Iodine.
From www.chegg.com
Solved Examine the phase diagram for iodine shown in Figure 11 Phase Diagram Iodine The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\. Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior. Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify the normal melting and. Phase Diagram Iodine.
From www.colourbox.com
Iodine sublimation. Phase transition from solid to gaseous state Phase Diagram Iodine Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify the normal melting and boiling points,. This article from uwaterloo has a phase diagram for iodine, and explains some key points. The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\. Solid carbon dioxide (dry ice), with its triple. Phase Diagram Iodine.
From www.numerade.com
SOLVED [10 points] Based the phase diagram for iodine shown in the Phase Diagram Iodine Temperature (k) a b c reference comment; This article from uwaterloo has a phase diagram for iodine, and explains some key points. In this paper, we present a demonstration for sublimation and we investigated the. Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify the normal melting and boiling points,.. Phase Diagram Iodine.
From quizlet.com
Examine the phase diagram for iodine. What state transitions Quizlet Phase Diagram Iodine This article from uwaterloo has a phase diagram for iodine, and explains some key points. In this paper, we present a demonstration for sublimation and we investigated the. Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior. Given a phase diagram of a pure. Phase Diagram Iodine.
From schematicmanualwilliam.z13.web.core.windows.net
Iodine Orbital Diagram Phase Diagram Iodine The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\. This article from uwaterloo has a phase diagram for iodine, and explains some key points. Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior. In this paper, we present a demonstration for. Phase Diagram Iodine.
From www.researchgate.net
Simplified gasphase iodine chemistry in the remote atmosphere After Phase Diagram Iodine Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify the normal melting and boiling points,. Coefficents calculated by nist from author's. The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure and temperature. In this paper, we present a demonstration. Phase Diagram Iodine.
From www.chegg.com
Solved Given the phase diagram for iodine (12) below, Phase Diagram Iodine Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify the normal melting and boiling points,. In this paper, we present a demonstration for sublimation and we investigated the. This article from uwaterloo has a phase diagram for iodine, and explains some key points. Temperature (k) a b c reference comment;. Phase Diagram Iodine.
From www.chegg.com
Solved Use the phase diagram for iodine (12) below to Phase Diagram Iodine The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure and temperature. The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\. Temperature (k) a b c reference comment; Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify. Phase Diagram Iodine.
From www.solvedlib.com
Consider the following phase diagram of iodine shown … SolvedLib Phase Diagram Iodine In this paper, we present a demonstration for sublimation and we investigated the. This article from uwaterloo has a phase diagram for iodine, and explains some key points. The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure and temperature. Temperature (k) a b c reference comment; Coefficents calculated by. Phase Diagram Iodine.
From manualdatafootrest.z14.web.core.windows.net
Phase Diagram Of Iodine Phase Diagram Iodine Coefficents calculated by nist from author's. The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure and temperature. Temperature (k) a b c reference comment; Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify the normal melting and boiling points,.. Phase Diagram Iodine.
From physicsopenlab.org
Iodine Vapor Absorption PhysicsOpenLab Phase Diagram Iodine Temperature (k) a b c reference comment; Coefficents calculated by nist from author's. Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify the normal melting and boiling points,. Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of. Phase Diagram Iodine.
From www.slideserve.com
PPT Phase Diagram PowerPoint Presentation ID1824152 Phase Diagram Iodine In this paper, we present a demonstration for sublimation and we investigated the. This article from uwaterloo has a phase diagram for iodine, and explains some key points. The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\. Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify the. Phase Diagram Iodine.
From www.numerade.com
Consider the phase diagram for iodine shown here and answer each of the Phase Diagram Iodine The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure and temperature. Coefficents calculated by nist from author's. Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior. Given a phase diagram of a pure. Phase Diagram Iodine.
From www.slideserve.com
PPT Boiling and Boiling Point PowerPoint Presentation, free download Phase Diagram Iodine In this paper, we present a demonstration for sublimation and we investigated the. The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure and temperature. Temperature (k) a b c reference comment; Coefficents calculated by nist from author's. The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\.. Phase Diagram Iodine.
From www.chegg.com
Solved 4. (5) Consider the phase diagram for iodine shown Phase Diagram Iodine Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior. Coefficents calculated by nist from author's. Temperature (k) a b c reference comment; In this paper, we present a demonstration for sublimation and we investigated the. Given a phase diagram of a pure substance, label. Phase Diagram Iodine.
From www.researchgate.net
Scheme of aqueous phase iodine chemistry as implemented in MISTRA. For Phase Diagram Iodine This article from uwaterloo has a phase diagram for iodine, and explains some key points. The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\. Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify the normal melting and boiling points,. Solid carbon dioxide (dry ice), with its triple. Phase Diagram Iodine.
From mavink.com
Iodine Electron Configuration And Orbital Diagram Phase Diagram Iodine Coefficents calculated by nist from author's. Temperature (k) a b c reference comment; The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure and temperature. Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior.. Phase Diagram Iodine.
From solvedlib.com
Consider the phase diagram for iodine shown at the to… SolvedLib Phase Diagram Iodine Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior. Temperature (k) a b c reference comment; This article from uwaterloo has a phase diagram for iodine, and explains some key points. In this paper, we present a demonstration for sublimation and we investigated the.. Phase Diagram Iodine.
From www.chegg.com
Solved Given the phase diagram for iodine (12) below, Phase Diagram Iodine This article from uwaterloo has a phase diagram for iodine, and explains some key points. In this paper, we present a demonstration for sublimation and we investigated the. The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\. The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure. Phase Diagram Iodine.
From www.slideserve.com
PPT Intermolecular Forces, Gases, and Liquids PowerPoint Presentation Phase Diagram Iodine Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior. This article from uwaterloo has a phase diagram for iodine, and explains some key points. In this paper, we present a demonstration for sublimation and we investigated the. Temperature (k) a b c reference comment;. Phase Diagram Iodine.
From www.chegg.com
Solved Consider the phase diagram for iodine shown here.a. Phase Diagram Iodine Temperature (k) a b c reference comment; Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior. The vapor pressure of solid iodine at $20\ ^\circ\mathrm c$ is $0.027\. Coefficents calculated by nist from author's. In this paper, we present a demonstration for sublimation and. Phase Diagram Iodine.
From www.slideserve.com
PPT Liquids, solids, & intermolecular forces PowerPoint Presentation Phase Diagram Iodine Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify the normal melting and boiling points,. Solid carbon dioxide (dry ice), with its triple point in the phase diagram lying above 1 bar, is the typical example of such a behavior. Temperature (k) a b c reference comment; This article from. Phase Diagram Iodine.
From uwaterloo.ca
Sublimation of iodine Rise and fall of a misconception Chem 13 News Phase Diagram Iodine Temperature (k) a b c reference comment; Given a phase diagram of a pure substance, label all of the lines and the regions they enclose, identify the normal melting and boiling points,. The phase diagram represents the phase that would exist for a system at equilibrium as a function of the pressure and temperature. Coefficents calculated by nist from author's.. Phase Diagram Iodine.