Collision Definition Theory . Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. The minimum kinetic energy required for. The rate of the reaction depends on the frequency of collisions. The collision theory is based on the. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates; A basic principal of collision theory is that, in order to react, molecules must collide. Define the concepts of activation energy and transition state;. This fundamental rule guides any analysis of an ordinary reaction mechanism. Collision theory states that for a reaction to take place, reactants must collide properly. The rate of reaction is equal to the frequency of collisions. For a chemical reaction to occur, the reactant molecules must collide with enough energy.
from www.sciencefacts.net
Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates; For a chemical reaction to occur, the reactant molecules must collide with enough energy. The minimum kinetic energy required for. This fundamental rule guides any analysis of an ordinary reaction mechanism. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Define the concepts of activation energy and transition state;. Collision theory states that for a reaction to take place, reactants must collide properly. A basic principal of collision theory is that, in order to react, molecules must collide. The rate of reaction is equal to the frequency of collisions. The collision theory is based on the.
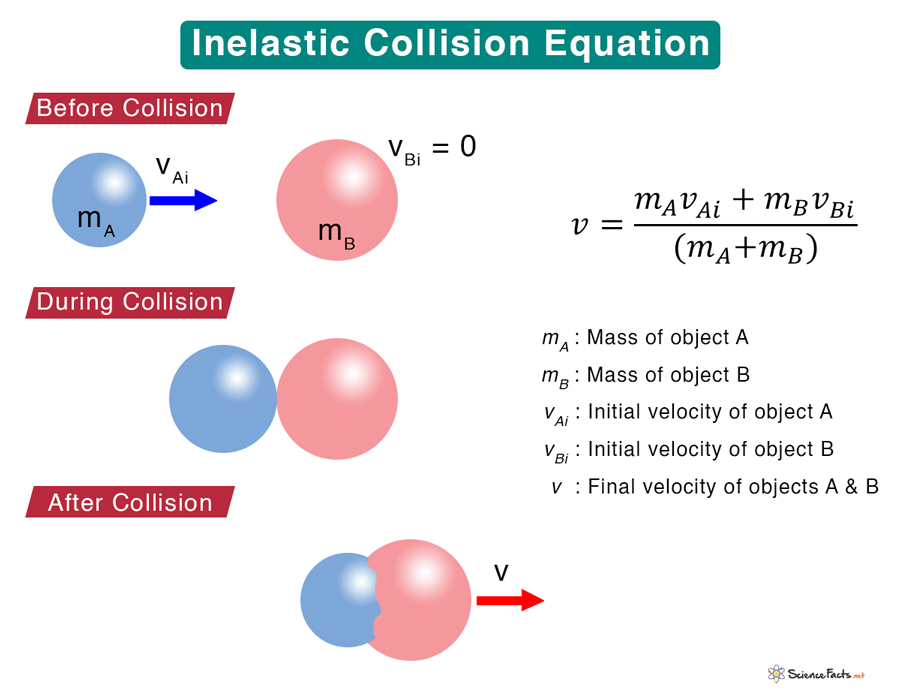
Inelastic Collision Definition, Formula, and Examples
Collision Definition Theory Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. For a chemical reaction to occur, the reactant molecules must collide with enough energy. This fundamental rule guides any analysis of an ordinary reaction mechanism. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates; Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. A basic principal of collision theory is that, in order to react, molecules must collide. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. The collision theory is based on the. The rate of the reaction depends on the frequency of collisions. Define the concepts of activation energy and transition state;. Collision theory states that for a reaction to take place, reactants must collide properly. The minimum kinetic energy required for. The rate of reaction is equal to the frequency of collisions.
From saylordotorg.github.io
The Collision Model of Chemical Collision Definition Theory This fundamental rule guides any analysis of an ordinary reaction mechanism. The collision theory is based on the. The minimum kinetic energy required for. Define the concepts of activation energy and transition state;. The rate of the reaction depends on the frequency of collisions. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration. Collision Definition Theory.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Collision Definition Theory The collision theory is based on the. This fundamental rule guides any analysis of an ordinary reaction mechanism. The minimum kinetic energy required for. The rate of the reaction depends on the frequency of collisions. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates; The rate of reaction is equal. Collision Definition Theory.
From www.slideserve.com
PPT Collision Theory and Reaction Rates PowerPoint Presentation, free Collision Definition Theory The minimum kinetic energy required for. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. The collision theory is based on the. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Define the concepts of activation energy and transition state;. Collision theory states that. Collision Definition Theory.
From studylib.net
Collision Theory and Potential Energy Diagrams Collision Definition Theory Define the concepts of activation energy and transition state;. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. The rate of reaction is equal to the frequency of collisions. For a chemical reaction to occur, the reactant molecules must collide with enough energy. Collision theory provides a qualitative explanation of chemical reactions and the rates. Collision Definition Theory.
From www.youtube.com
Collision Theory YouTube Collision Definition Theory The minimum kinetic energy required for. A basic principal of collision theory is that, in order to react, molecules must collide. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. Collision theory states that for a reaction to take place, reactants must collide properly. For a chemical reaction to occur, the reactant molecules. Collision Definition Theory.
From www.ck12.org
Collision Theory CK12 Foundation Collision Definition Theory A basic principal of collision theory is that, in order to react, molecules must collide. The rate of reaction is equal to the frequency of collisions. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Define the concepts of activation energy and transition state;. The minimum kinetic energy required for. Collision theory states that for. Collision Definition Theory.
From wiringfixsprang.z19.web.core.windows.net
Parts Of Collision Theory Collision Definition Theory Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates; The collision theory is based on the. Define the concepts of activation energy and transition state;. For a chemical reaction to occur, the reactant molecules must collide with enough energy. The minimum kinetic energy required for. The rate of the reaction. Collision Definition Theory.
From www.slideserve.com
PPT Ch. 18—Reaction Rates and Equilibrium PowerPoint Presentation Collision Definition Theory The minimum kinetic energy required for. The rate of the reaction depends on the frequency of collisions. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates; Collision theory states that for a reaction to take place, reactants must collide properly. The collision theory is based on the. Collision theory states. Collision Definition Theory.
From www.savemyexams.com
Collision Theory Cambridge O Level Chemistry Revision Notes 2023 Collision Definition Theory The rate of reaction is equal to the frequency of collisions. Collision theory states that for a reaction to take place, reactants must collide properly. The collision theory is based on the. This fundamental rule guides any analysis of an ordinary reaction mechanism. For a chemical reaction to occur, the reactant molecules must collide with enough energy. A basic principal. Collision Definition Theory.
From www.slideshare.net
Collision Theory Collision Definition Theory Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. The minimum kinetic energy required for. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Define the concepts of activation energy and transition state;. The collision theory is based on the. Collision theory provides a qualitative explanation. Collision Definition Theory.
From nsmn1.uh.edu
Definition of Collisions Collision Definition Theory Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Define the concepts of activation energy and transition state;. The rate of reaction is equal to the frequency of collisions. For a chemical reaction to occur, the reactant molecules must collide with enough energy. The minimum kinetic energy required for. A basic principal of collision theory. Collision Definition Theory.
From www.slideserve.com
PPT Chapters 16, 17, 18 PowerPoint Presentation, free download ID Collision Definition Theory The collision theory is based on the. Define the concepts of activation energy and transition state;. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. This fundamental rule guides any analysis of an ordinary reaction mechanism. Use the postulates of collision theory to explain the effects of physical state, temperature, and. Collision Definition Theory.
From studyfinder.org
Understanding Collision Theory Unraveling Gizmo Answers Collision Definition Theory Define the concepts of activation energy and transition state;. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Collision theory states that for a reaction to take place, reactants must collide properly. This fundamental rule guides any analysis of an ordinary reaction mechanism. A basic principal of collision theory is that,. Collision Definition Theory.
From www.sciencefacts.net
Inelastic Collision Definition, Formula, and Examples Collision Definition Theory Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. The minimum kinetic energy required for. For a chemical reaction to occur, the reactant molecules must collide with enough energy. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. The rate of reaction is equal to the. Collision Definition Theory.
From www.sciencefacts.net
Inelastic Collision Definition, Formula, and Examples Collision Definition Theory The rate of the reaction depends on the frequency of collisions. Collision theory states that for a reaction to take place, reactants must collide properly. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. The minimum kinetic. Collision Definition Theory.
From www.sciencefacts.net
Elastic Collision Definition, Formula, and Examples Collision Definition Theory Collision theory states that for a reaction to take place, reactants must collide properly. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. The minimum kinetic energy required for. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. The collision theory is based on. Collision Definition Theory.
From www.coursehero.com
Collision Theory General Chemistry I Course Hero Collision Definition Theory This fundamental rule guides any analysis of an ordinary reaction mechanism. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates; Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Collision theory provides a qualitative explanation of chemical reactions and the rates. Collision Definition Theory.
From lessonmariann.z21.web.core.windows.net
Collision Definition Science Collision Definition Theory The minimum kinetic energy required for. This fundamental rule guides any analysis of an ordinary reaction mechanism. The collision theory is based on the. The rate of reaction is equal to the frequency of collisions. The rate of the reaction depends on the frequency of collisions. For a chemical reaction to occur, the reactant molecules must collide with enough energy.. Collision Definition Theory.
From www.youtube.com
6.1 Collision theory (SL) YouTube Collision Definition Theory The collision theory is based on the. Collision theory states that for a reaction to take place, reactants must collide properly. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. The rate of reaction is equal to the frequency of collisions. Define the concepts of activation energy and transition state;. Collision theory provides a qualitative. Collision Definition Theory.
From www.geeksforgeeks.org
Elastic Collision Definition, Formula, and Example Collision Definition Theory Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates; This fundamental rule guides any analysis of an ordinary reaction mechanism. The rate of the reaction depends on the frequency of collisions. Collision theory states that for a reaction to take place, reactants must collide properly. For a chemical reaction to. Collision Definition Theory.
From www.slideserve.com
PPT Collisions and Momentum PowerPoint Presentation, free download Collision Definition Theory Define the concepts of activation energy and transition state;. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates; For a chemical reaction to occur, the reactant molecules must collide with enough energy. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory states that. Collision Definition Theory.
From www.slideserve.com
PPT Chapter 9 PowerPoint Presentation, free download ID5697293 Collision Definition Theory A basic principal of collision theory is that, in order to react, molecules must collide. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. The rate of reaction is equal to the frequency of collisions. Collision theory states that for a reaction to take place, reactants must collide properly. For a. Collision Definition Theory.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID6545697 Collision Definition Theory A basic principal of collision theory is that, in order to react, molecules must collide. For a chemical reaction to occur, the reactant molecules must collide with enough energy. The minimum kinetic energy required for. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Collision theory, theory used to predict the. Collision Definition Theory.
From www.pinterest.co.uk
Collision Theory Easy Science Collision theory, Theories, Theory Collision Definition Theory The collision theory is based on the. For a chemical reaction to occur, the reactant molecules must collide with enough energy. This fundamental rule guides any analysis of an ordinary reaction mechanism. The rate of reaction is equal to the frequency of collisions. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on. Collision Definition Theory.
From study.com
Elastic Collision Definition, Characteristics & Examples Lesson Collision Definition Theory A basic principal of collision theory is that, in order to react, molecules must collide. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. This fundamental rule guides any analysis of an ordinary reaction mechanism. The minimum kinetic energy required for. Collision theory states that for a chemical reaction to occur, the reacting particles must. Collision Definition Theory.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID2428843 Collision Definition Theory Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates; Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. The minimum kinetic energy required for. The rate of the reaction. Collision Definition Theory.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Collision Definition Theory Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. Collision theory states that for a reaction to take place, reactants must collide properly. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. The minimum kinetic energy required for. The collision theory is based on. Collision Definition Theory.
From www.youtube.com
Collision Theory and How to Ensure Effective Collisions in Chemical Collision Definition Theory The rate of the reaction depends on the frequency of collisions. This fundamental rule guides any analysis of an ordinary reaction mechanism. Define the concepts of activation energy and transition state;. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. The rate of reaction is equal to the frequency of collisions. Use the. Collision Definition Theory.
From byjus.com
Collision Theory Definition, Explanation, Activation energy, Arrhenius Collision Definition Theory Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates; This fundamental rule guides any analysis of an ordinary reaction mechanism. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. The minimum kinetic energy required for. Collision theory states that for a chemical reaction to occur,. Collision Definition Theory.
From www.slideserve.com
PPT Earth and Moon Statistics PowerPoint Presentation, free download Collision Definition Theory This fundamental rule guides any analysis of an ordinary reaction mechanism. Collision theory states that for a reaction to take place, reactants must collide properly. Define the concepts of activation energy and transition state;. Collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. The minimum kinetic energy required for. Collision theory, theory used. Collision Definition Theory.
From www.aquaportail.com
Théorie des collisions définition et explications Collision Definition Theory The collision theory is based on the. This fundamental rule guides any analysis of an ordinary reaction mechanism. A basic principal of collision theory is that, in order to react, molecules must collide. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Use the postulates of collision theory to explain the. Collision Definition Theory.
From www.slideserve.com
PPT Collision Theory PowerPoint Presentation, free download ID3873529 Collision Definition Theory Collision theory states that for a reaction to take place, reactants must collide properly. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates; Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. The rate of reaction is equal to the frequency. Collision Definition Theory.
From www.pinterest.com
Collision Theory Collision theory, Teaching chemistry, Chemistry lessons Collision Definition Theory The rate of the reaction depends on the frequency of collisions. For a chemical reaction to occur, the reactant molecules must collide with enough energy. This fundamental rule guides any analysis of an ordinary reaction mechanism. The collision theory is based on the. A basic principal of collision theory is that, in order to react, molecules must collide. Use the. Collision Definition Theory.
From mmerevise.co.uk
Collision Theory and Reaction Rates MME Collision Definition Theory Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. This fundamental rule guides any analysis of an ordinary reaction mechanism. The rate of the reaction depends on the frequency of collisions. The rate of reaction is equal to the frequency of collisions. Collision theory states that for a chemical reaction to occur, the reacting particles. Collision Definition Theory.
From www.youtube.com
introduction to collision theory YouTube Collision Definition Theory For a chemical reaction to occur, the reactant molecules must collide with enough energy. A basic principal of collision theory is that, in order to react, molecules must collide. This fundamental rule guides any analysis of an ordinary reaction mechanism. The rate of the reaction depends on the frequency of collisions. Use the postulates of collision theory to explain the. Collision Definition Theory.