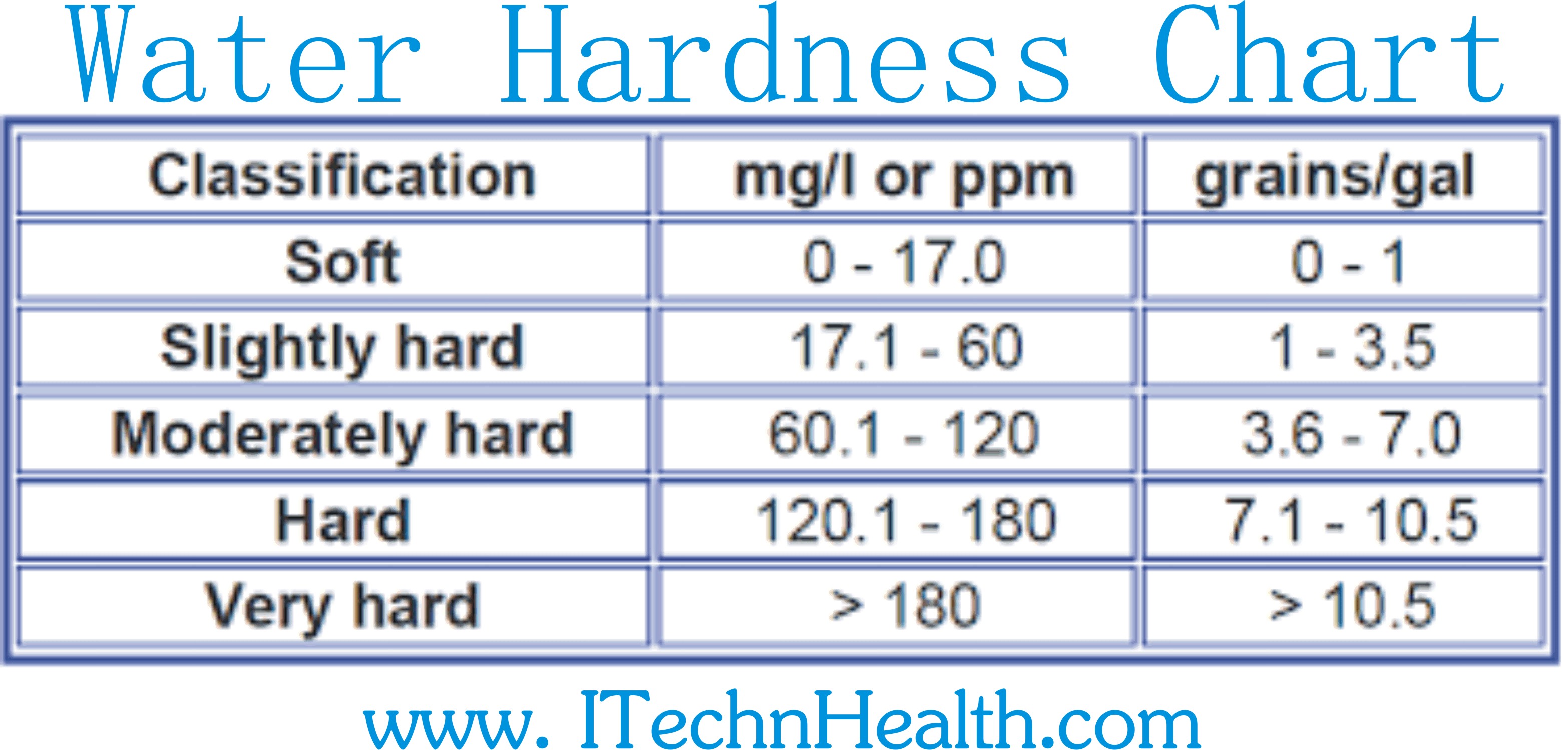
How To Calculate Water Hardness Chemistry . In this experiment, you will determine the “hardness” of a water sample by complexometric titration. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: The water hardness calculation is based on the sum of the concentrations of calcium and magnesium ions,. How it calculates the results. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). Analytes other than acids and bases can also be measured via titrimetry. For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. Make a standard solution of edta. The base equation we use to measure total hardness is. Calculating total hardness of water takes into account the dissolution of all elements that contain calcium and magnesium atoms.
from itechnhealth.com
Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). In this experiment, you will determine the “hardness” of a water sample by complexometric titration. For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. The water hardness calculation is based on the sum of the concentrations of calcium and magnesium ions,. Make a standard solution of edta. Analytes other than acids and bases can also be measured via titrimetry. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: The base equation we use to measure total hardness is. How it calculates the results. Calculating total hardness of water takes into account the dissolution of all elements that contain calcium and magnesium atoms.
How Do You Know If You Have Hard Water
How To Calculate Water Hardness Chemistry The water hardness calculation is based on the sum of the concentrations of calcium and magnesium ions,. How it calculates the results. Make a standard solution of edta. Analytes other than acids and bases can also be measured via titrimetry. In this experiment, you will determine the “hardness” of a water sample by complexometric titration. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. Calculating total hardness of water takes into account the dissolution of all elements that contain calcium and magnesium atoms. The base equation we use to measure total hardness is. The water hardness calculation is based on the sum of the concentrations of calcium and magnesium ions,.
From www.youtube.com
Numerical Hardness of water YouTube How To Calculate Water Hardness Chemistry In this experiment, you will determine the “hardness” of a water sample by complexometric titration. Make a standard solution of edta. The water hardness calculation is based on the sum of the concentrations of calcium and magnesium ions,. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). How it calculates the results. There are. How To Calculate Water Hardness Chemistry.
From www.youtube.com
Hardness of Water//Chemistry//Class 10 YouTube How To Calculate Water Hardness Chemistry Analytes other than acids and bases can also be measured via titrimetry. Calculating total hardness of water takes into account the dissolution of all elements that contain calcium and magnesium atoms. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). The base equation we use to measure total hardness is. In this experiment, you. How To Calculate Water Hardness Chemistry.
From mavink.com
Water Hardness Conversion Chart How To Calculate Water Hardness Chemistry Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). Analytes other than acids and bases can also be measured via titrimetry. In this experiment, you will determine the “hardness” of a water sample by complexometric titration. The base equation we use to measure total hardness is. How it calculates the results. Make a standard. How To Calculate Water Hardness Chemistry.
From www.youtube.com
Calculation of Water Hardness Numerical and practice problem Part1 How To Calculate Water Hardness Chemistry For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: In this experiment, you will determine the “hardness” of a water sample by complexometric titration. The water hardness calculation is based on. How To Calculate Water Hardness Chemistry.
From waterfilterguru.com
Water Hardness Calculator Easily Determine Total Hardness How To Calculate Water Hardness Chemistry Analytes other than acids and bases can also be measured via titrimetry. In this experiment, you will determine the “hardness” of a water sample by complexometric titration. How it calculates the results. Make a standard solution of edta. Calculating total hardness of water takes into account the dissolution of all elements that contain calcium and magnesium atoms. Water hardness can. How To Calculate Water Hardness Chemistry.
From waternitylab.com
Water Hardness Scale GPG, mmol/L, PPM Chart How To Calculate Water Hardness Chemistry In this experiment, you will determine the “hardness” of a water sample by complexometric titration. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). The base equation we use to measure total hardness is. Make a standard solution of edta. The water hardness calculation is based on the sum of the concentrations of calcium. How To Calculate Water Hardness Chemistry.
From studylib.net
Hardness of Water Presentation How To Calculate Water Hardness Chemistry Analytes other than acids and bases can also be measured via titrimetry. For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. In this experiment, you will determine the “hardness” of a water sample by complexometric titration. How it calculates the results. The water hardness calculation is based on the sum of the concentrations of. How To Calculate Water Hardness Chemistry.
From klahkfosd.blob.core.windows.net
Water Hardness Unit Converter at Lillie Cornell blog How To Calculate Water Hardness Chemistry In this experiment, you will determine the “hardness” of a water sample by complexometric titration. How it calculates the results. For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. The water hardness calculation is based on the sum of the concentrations of calcium and magnesium ions,. Analytes other than acids and bases can also. How To Calculate Water Hardness Chemistry.
From pt.slideshare.net
Water hardness edta How To Calculate Water Hardness Chemistry Make a standard solution of edta. In this experiment, you will determine the “hardness” of a water sample by complexometric titration. Analytes other than acids and bases can also be measured via titrimetry. How it calculates the results. The base equation we use to measure total hardness is. There are 3 steps to determining the concentration of calcium and magnesium. How To Calculate Water Hardness Chemistry.
From mavink.com
Water Hardness Conversion Chart How To Calculate Water Hardness Chemistry Make a standard solution of edta. Calculating total hardness of water takes into account the dissolution of all elements that contain calcium and magnesium atoms. How it calculates the results. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). Analytes other than acids and bases can also be measured via titrimetry. There are 3. How To Calculate Water Hardness Chemistry.
From mavink.com
Water Hardness Conversion Chart How To Calculate Water Hardness Chemistry For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. Analytes other than acids and bases can also be measured via titrimetry. How it calculates the results. The base equation we use to measure total hardness is. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the. How To Calculate Water Hardness Chemistry.
From www.youtube.com
Total Water Hardness using EDTA Titration YouTube How To Calculate Water Hardness Chemistry In this experiment, you will determine the “hardness” of a water sample by complexometric titration. How it calculates the results. For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. The water hardness calculation is based on the sum of the concentrations of calcium and magnesium ions,. The base equation we use to measure total. How To Calculate Water Hardness Chemistry.
From www.youtube.com
How to calculate the temporary & permanent hardness in Water Sample How To Calculate Water Hardness Chemistry The water hardness calculation is based on the sum of the concentrations of calcium and magnesium ions,. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. There are 3 steps to determining the concentration of calcium and magnesium ions. How To Calculate Water Hardness Chemistry.
From www.scribd.com
Water Hardness Conversion Table PDF Units Of Measurement Chemistry How To Calculate Water Hardness Chemistry In this experiment, you will determine the “hardness” of a water sample by complexometric titration. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: How it calculates the results. Analytes other than acids and bases can also be measured via titrimetry. The water hardness calculation is. How To Calculate Water Hardness Chemistry.
From www.youtube.com
How to calculate hardness of water YouTube How To Calculate Water Hardness Chemistry Make a standard solution of edta. Calculating total hardness of water takes into account the dissolution of all elements that contain calcium and magnesium atoms. In this experiment, you will determine the “hardness” of a water sample by complexometric titration. Analytes other than acids and bases can also be measured via titrimetry. The base equation we use to measure total. How To Calculate Water Hardness Chemistry.
From www.youtube.com
Units of Hardness of water and numericals Part2 YouTube How To Calculate Water Hardness Chemistry There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). In this experiment, you will determine the “hardness” of a water sample by complexometric titration. The base equation we use to measure. How To Calculate Water Hardness Chemistry.
From graingerrevise.blogspot.com
Chemistry Revision C6f Hardness of Water How To Calculate Water Hardness Chemistry Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). The water hardness calculation is based on the sum of the concentrations of calcium and magnesium ions,. Calculating total hardness of water takes into account the dissolution of all elements that contain calcium and magnesium atoms. How it calculates the results. There are 3 steps. How To Calculate Water Hardness Chemistry.
From www.youtube.com
Units of Hardness of water // Engg Chemistry YouTube How To Calculate Water Hardness Chemistry Analytes other than acids and bases can also be measured via titrimetry. For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. Make a standard solution of edta. Calculating total hardness of water takes into account the dissolution of all elements that contain calcium and magnesium atoms. In this experiment, you will determine the “hardness”. How To Calculate Water Hardness Chemistry.
From www.numerade.com
A water sample contains 200 mg of CaSO4 per liter. Calculate the How To Calculate Water Hardness Chemistry Make a standard solution of edta. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). Calculating total hardness of water takes into account the dissolution of all elements that contain calcium and magnesium atoms. The base equation we use to measure total hardness is. There are 3 steps to determining the concentration of calcium. How To Calculate Water Hardness Chemistry.
From www.youtube.com
To calculate total hardness of a given water sample BSC 2 year How To Calculate Water Hardness Chemistry The water hardness calculation is based on the sum of the concentrations of calcium and magnesium ions,. Calculating total hardness of water takes into account the dissolution of all elements that contain calcium and magnesium atoms. Make a standard solution of edta. Analytes other than acids and bases can also be measured via titrimetry. Water hardness can be readily determined. How To Calculate Water Hardness Chemistry.
From www.youtube.com
Hardness of Water Solved Examples Environmental Engineering YouTube How To Calculate Water Hardness Chemistry The water hardness calculation is based on the sum of the concentrations of calcium and magnesium ions,. How it calculates the results. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). In this experiment, you will determine the “hardness” of a water sample by complexometric titration. Make a standard solution of edta. For epa. How To Calculate Water Hardness Chemistry.
From www.youtube.com
Total Hardness Calculation Water Hardness Module 3, Topic 2 Water How To Calculate Water Hardness Chemistry For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: Calculating total hardness of water takes into account the dissolution of all elements that contain calcium and magnesium atoms. Analytes other than. How To Calculate Water Hardness Chemistry.
From www.youtube.com
General Chemistry Lecture 233 Some Problems of Hardness of Water How To Calculate Water Hardness Chemistry Make a standard solution of edta. Analytes other than acids and bases can also be measured via titrimetry. The base equation we use to measure total hardness is. For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. In this experiment, you will determine the “hardness” of a water sample by complexometric titration. The water. How To Calculate Water Hardness Chemistry.
From www.culligan-mechanicsburg.com
StepbyStep Guide Measuring the Hardness of Your Water How To Calculate Water Hardness Chemistry The base equation we use to measure total hardness is. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration. How To Calculate Water Hardness Chemistry.
From www.youtube.com
Numerical on EDTA method I How to calculate Total, Temporary How To Calculate Water Hardness Chemistry For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: Analytes other than acids and bases can also be measured via titrimetry. Make a standard solution of edta. How it calculates the. How To Calculate Water Hardness Chemistry.
From exonzmvvd.blob.core.windows.net
Water Hardness Test Reaction at Anne Howard blog How To Calculate Water Hardness Chemistry In this experiment, you will determine the “hardness” of a water sample by complexometric titration. The base equation we use to measure total hardness is. The water hardness calculation is based on the sum of the concentrations of calcium and magnesium ions,. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the. How To Calculate Water Hardness Chemistry.
From www.youtube.com
Water Hardness (EDTA) Titration Calculations Example YouTube How To Calculate Water Hardness Chemistry How it calculates the results. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: Make a standard solution of edta. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). Analytes other than acids and bases can also be measured via. How To Calculate Water Hardness Chemistry.
From waterfilterguru.com
What is the Ideal Water Hardness Level? Water Filter Guru How To Calculate Water Hardness Chemistry Analytes other than acids and bases can also be measured via titrimetry. In this experiment, you will determine the “hardness” of a water sample by complexometric titration. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: For epa method 130.2 eriochrome black t (ebt) is used. How To Calculate Water Hardness Chemistry.
From www.youtube.com
Estimation of Hardness of water calculation Viva Voce Chemistry How To Calculate Water Hardness Chemistry Make a standard solution of edta. The base equation we use to measure total hardness is. Calculating total hardness of water takes into account the dissolution of all elements that contain calcium and magnesium atoms. For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. Analytes other than acids and bases can also be measured. How To Calculate Water Hardness Chemistry.
From www.waterfiltermag.com
Water Hardness Definition, Example, and Explanation How To Calculate Water Hardness Chemistry The water hardness calculation is based on the sum of the concentrations of calcium and magnesium ions,. For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. In this experiment, you will determine the “hardness” of a water sample by complexometric titration. There are 3 steps to determining the concentration of calcium and magnesium ions. How To Calculate Water Hardness Chemistry.
From www.youtube.com
How to calculate temporary and permanent hardness of water? YouTube How To Calculate Water Hardness Chemistry The water hardness calculation is based on the sum of the concentrations of calcium and magnesium ions,. There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with edta: Analytes other than acids and bases can also be measured via titrimetry. In this experiment, you will determine the “hardness”. How To Calculate Water Hardness Chemistry.
From www.youtube.com
Numerical Problem on Hardness of Water by EDTA Method YouTube How To Calculate Water Hardness Chemistry Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. In this experiment, you will determine the “hardness” of a water sample by complexometric titration. Calculating total hardness of water takes into account the dissolution of all elements that contain. How To Calculate Water Hardness Chemistry.
From itechnhealth.com
How Do You Know If You Have Hard Water How To Calculate Water Hardness Chemistry For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. In this experiment, you will determine the “hardness” of a water sample by complexometric titration. The base equation we use to measure total hardness is. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). The water hardness calculation is. How To Calculate Water Hardness Chemistry.
From www.youtube.com
How to determine hardness of water by EDTA method? (Procedure and How To Calculate Water Hardness Chemistry The base equation we use to measure total hardness is. How it calculates the results. Analytes other than acids and bases can also be measured via titrimetry. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the. How To Calculate Water Hardness Chemistry.
From netsolwater.com
How to Measure of Hardness in Water Netsol Water How To Calculate Water Hardness Chemistry Make a standard solution of edta. Analytes other than acids and bases can also be measured via titrimetry. For epa method 130.2 eriochrome black t (ebt) is used to determine total water hardness. Water hardness can be readily determined by titration with the chelating agent edta (ethylenediaminetetraacetic acid). In this experiment, you will determine the “hardness” of a water sample. How To Calculate Water Hardness Chemistry.