Dilute Solution How To . This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume of solution, how to prepare a. Learn how to dilute and concentrate solutions. Of course, the resulting solution is thoroughly mixed so as to. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is also a common. The solution on the right is more dilute because the copper nitrate is dissolved in more solvent. To dilute a solution means to add more solvent without the addition of more solute. The solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary volume of a diluted solution. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. A concentrated solution contains a. Dilution is the addition of.
from chem.libretexts.org
The solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary volume of a diluted solution. Dilution is the addition of. A concentrated solution contains a. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Of course, the resulting solution is thoroughly mixed so as to. Learn how to dilute and concentrate solutions. Dilution is also a common. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. To dilute a solution means to add more solvent without the addition of more solute.
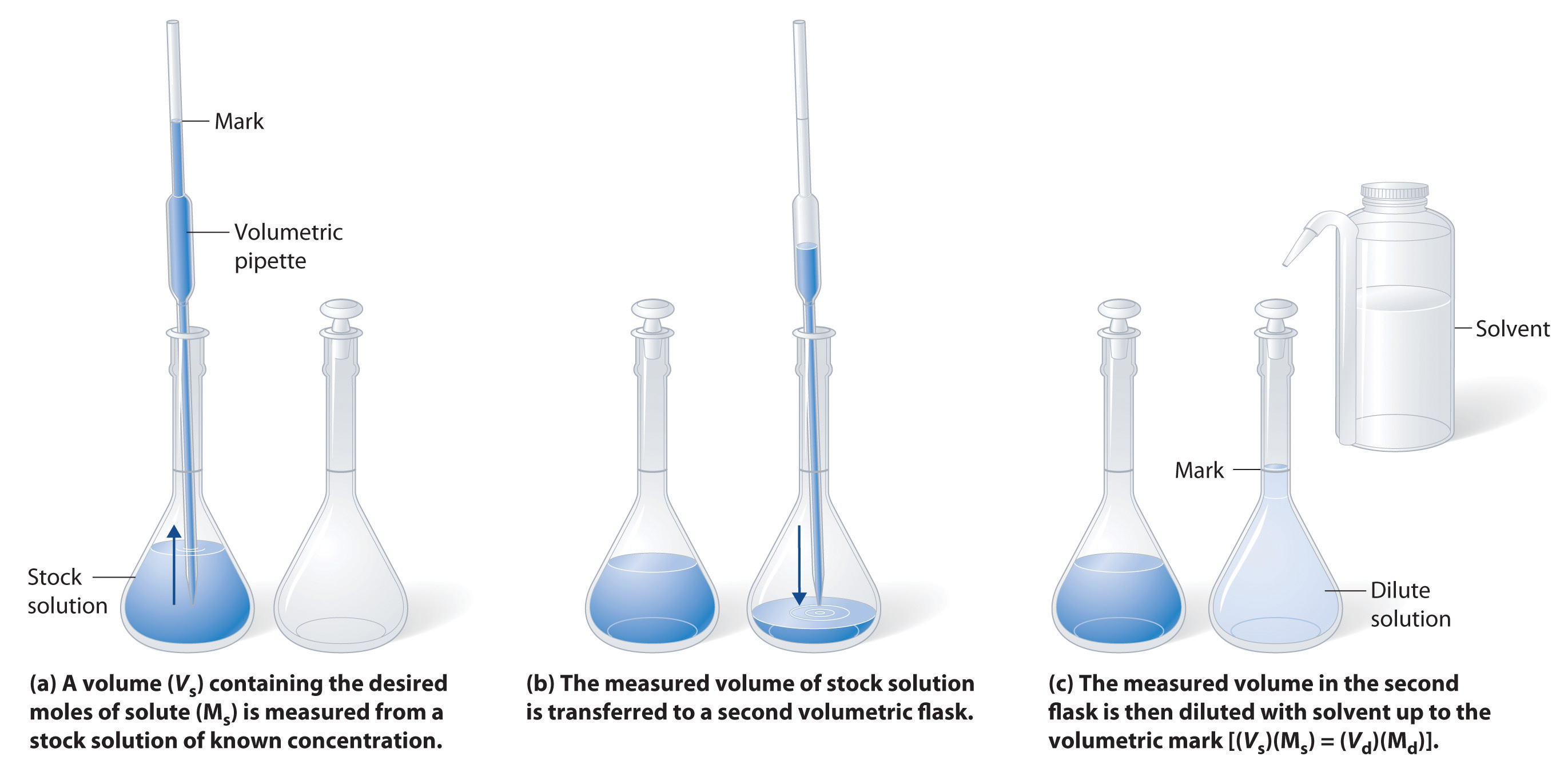
5.2 Solutions and Dilutions Chemistry LibreTexts
Dilute Solution How To Learn how to dilute and concentrate solutions. Of course, the resulting solution is thoroughly mixed so as to. To dilute a solution means to add more solvent without the addition of more solute. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume of solution, how to prepare a. The solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Learn how to dilute and concentrate solutions. The solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary volume of a diluted solution. A concentrated solution contains a. Dilution is the addition of. Dilution is also a common. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent.
From www.youtube.com
What is Dilute Solution? Chemistry YouTube Dilute Solution How To To dilute a solution means to add more solvent without the addition of more solute. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume of solution, how to prepare a. Dilution is the addition of. The solution on the right is more dilute because the copper. Dilute Solution How To.
From polepride.weebly.com
How to prepare dilute ammonia solution polepride Dilute Solution How To A concentrated solution contains a. Dilution is also a common. Dilution is the addition of. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. The solution on the right is more dilute because the copper nitrate is dissolved in more solvent. To dilute a solution means to add. Dilute Solution How To.
From socratic.org
mL of solution has a concentration of 3.0 M. How much water needs to be Dilute Solution How To This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Dilution is the addition of. Of course, the resulting solution is thoroughly mixed so as to. The solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Dilution is also a common. A. Dilute Solution How To.
From www.youtube.com
How to prepare dilute solution of Sulphuric Acid (H2SO4) in laboratory Dilute Solution How To A concentrated solution contains a. Of course, the resulting solution is thoroughly mixed so as to. To dilute a solution means to add more solvent without the addition of more solute. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is the addition of. The solution dilution calculator will. Dilute Solution How To.
From studylib.net
Dilutions Dilute Solution How To Learn how to dilute and concentrate solutions. The solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary volume of a diluted solution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. A concentrated solution contains a. To dilute a solution. Dilute Solution How To.
From youtube.com
How to Dilute a Solution YouTube Dilute Solution How To The solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary volume of a diluted solution. To dilute a solution means to add more solvent without the addition of more solute. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution. Dilute Solution How To.
From www.medicine.mcgill.ca
Serial Dilutions Dilute Solution How To Dilution is also a common. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Learn how to dilute and concentrate solutions. The solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary volume of a diluted solution. Dilution is the addition. Dilute Solution How To.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilute Solution How To This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Dilution is also a common. To dilute a solution means to add more solvent without the addition of more solute. The solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain. Dilute Solution How To.
From www.vrogue.co
What Is Serial Dilution Method And How To Calculate S vrogue.co Dilute Solution How To Dilution is also a common. Of course, the resulting solution is thoroughly mixed so as to. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Learn how to dilute and concentrate solutions. The solution on the right is more dilute because the copper nitrate is dissolved in more. Dilute Solution How To.
From www.pinterest.com
Example of a Serial Dilution Dilute Solution How To The solution on the right is more dilute because the copper nitrate is dissolved in more solvent. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume of solution, how to prepare a. To dilute a solution means to add more solvent without the addition of more. Dilute Solution How To.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilute Solution How To A concentrated solution contains a. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Of course, the resulting solution is thoroughly mixed so as to. To dilute a solution means to add more solvent without the addition of more solute. Often, a worker will need to change the concentration of. Dilute Solution How To.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Dilute Solution How To Of course, the resulting solution is thoroughly mixed so as to. The solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary volume of a diluted solution. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Dilution is. Dilute Solution How To.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilute Solution How To Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Learn how to dilute and concentrate solutions. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. The solution on the right is more dilute because the copper nitrate is dissolved in more. Dilute Solution How To.
From laxenian.weebly.com
How To Dilute A Solution laxenian Dilute Solution How To The solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Dilution is also a common. Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. This guide will describe the process for preparing aqueous solutions, including how to calculate the. Dilute Solution How To.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilute Solution How To To dilute a solution means to add more solvent without the addition of more solute. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of. A concentrated solution contains a. Learn how to dilute and concentrate solutions. This guide will describe the process for preparing aqueous solutions,. Dilute Solution How To.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Solution How To The solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary volume of a diluted solution. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume of solution, how to prepare a. Dilution is the addition of.. Dilute Solution How To.
From slidetodoc.com
WATER AND SOLUTIONS A solution is a mixture Dilute Solution How To Dilution is also a common. The solution on the right is more dilute because the copper nitrate is dissolved in more solvent. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Learn how to dilute and concentrate solutions. The solution dilution calculator will calculate for you how to dilute a. Dilute Solution How To.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Solution How To Learn how to dilute and concentrate solutions. The solution on the right is more dilute because the copper nitrate is dissolved in more solvent. A concentrated solution contains a. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Dilution is the addition of. Of course, the resulting solution. Dilute Solution How To.
From www.youtube.com
Dilution, solution, ratio, proportion (laboratory. calculations) YouTube Dilute Solution How To The solution on the right is more dilute because the copper nitrate is dissolved in more solvent. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Learn how to dilute and concentrate solutions. This guide will describe the process for preparing aqueous solutions, including how to calculate the. Dilute Solution How To.
From study.com
How to Dilute a Strong Acid Solution to a Given pH Chemistry Dilute Solution How To A concentrated solution contains a. Of course, the resulting solution is thoroughly mixed so as to. Learn how to dilute and concentrate solutions. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent.. Dilute Solution How To.
From www.shutterstock.com
228 Dilute Solution Concentrated Solution Images, Stock Photos Dilute Solution How To The solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary volume of a diluted solution. Learn how to dilute and concentrate solutions. The solution on the right is more dilute because the copper nitrate is dissolved in more solvent. To dilute a solution means to add more solvent without. Dilute Solution How To.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Solution How To Dilution is also a common. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Learn how to dilute and concentrate solutions. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is the addition of. To dilute a solution means to. Dilute Solution How To.
From www.youtube.com
Dilute and Concentrated Solution YouTube Dilute Solution How To Of course, the resulting solution is thoroughly mixed so as to. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. A concentrated solution contains a. The solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary volume of. Dilute Solution How To.
From brainly.in
how can a solution be dilute or concentrated Brainly.in Dilute Solution How To A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to. Dilute Solution How To.
From ar.inspiredpencil.com
Dilute Solution Diagram Dilute Solution How To Learn how to dilute and concentrate solutions. The solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Of course, the resulting solution is thoroughly mixed so as to. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The solution dilution calculator will calculate for. Dilute Solution How To.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilute Solution How To A concentrated solution contains a. Dilution is the addition of. Learn how to dilute and concentrate solutions. Dilution is also a common. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume of solution, how to prepare a. This video and written tutorial will guide you through. Dilute Solution How To.
From www.pinterest.com.mx
Dilution when solvent is added to dilute a solution, the number of Dilute Solution How To To dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is thoroughly mixed so as to. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. The solution dilution calculator will calculate for you how to dilute a stock solution of. Dilute Solution How To.
From www.researchgate.net
How can I dilute aqueous Glutaraldehyde solution in water ? ResearchGate Dilute Solution How To Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Dilution is also a common. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution. Dilute Solution How To.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilute Solution How To A concentrated solution contains a. Of course, the resulting solution is thoroughly mixed so as to. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume of solution, how to prepare a. The solution on the right is more dilute because the copper nitrate is dissolved in. Dilute Solution How To.
From www.youtube.com
How to dilute concentrated solution Dilution of Solution Example Dilute Solution How To The solution on the right is more dilute because the copper nitrate is dissolved in more solvent. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume of solution, how to prepare a. The solution dilution calculator will calculate for you how to dilute a stock solution. Dilute Solution How To.
From www.slideserve.com
PPT What is osmosis? PowerPoint Presentation, free download ID2712925 Dilute Solution How To This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount of solute for a given volume of solution, how to prepare a. A concentrated solution contains a. Dilution is also a common. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor.. Dilute Solution How To.
From www.slideserve.com
PPT Serial Dilution PowerPoint Presentation, free download ID3119682 Dilute Solution How To Often, a worker will need to change the concentration of a solution by changing the amount of solvent. To dilute a solution means to add more solvent without the addition of more solute. Dilution is also a common. Learn how to dilute and concentrate solutions. This video and written tutorial will guide you through how to make a dilution and. Dilute Solution How To.
From www.slideserve.com
PPT Concentration and Dilution of Solutions PowerPoint Presentation Dilute Solution How To Dilution is the addition of. Of course, the resulting solution is thoroughly mixed so as to. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Learn how to dilute and concentrate solutions. The solution on the right is more dilute because the copper nitrate is dissolved in more. Dilute Solution How To.
From www.osmosis.org
Molarity and dilutions Vídeo, Anatomía & Definición Osmosis Dilute Solution How To Dilution is the addition of. A concentrated solution contains a. Dilution is also a common. Learn how to dilute and concentrate solutions. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. The solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain. Dilute Solution How To.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Dilute Solution How To The solution on the right is more dilute because the copper nitrate is dissolved in more solvent. The solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain an arbitrary volume of a diluted solution. This guide will describe the process for preparing aqueous solutions, including how to calculate the appropriate amount. Dilute Solution How To.