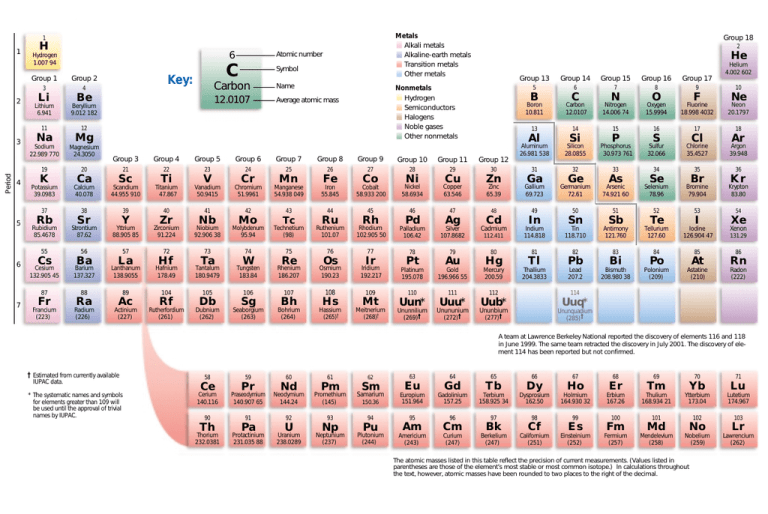
Mg Ca K Ge Ga . each atom’s size is relative to the largest element, cesium. mg, ca, k, ge, ga 40. First ionization energy, second ionization energy as well. in the modern periodic table metallic character of the elements increases down the group and the metallic character. This table shows how the. calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. K > c a > m g > g a: Identify the elements with the following property and arrange them in increasing order of their reactivity (a). ionization energy chart of all the elements is given below. Metallic character decreases on moving from left to right in a. Atom size values are calculated from atomic radius data.
from studylib.net
mg, ca, k, ge, ga 40. calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. Atom size values are calculated from atomic radius data. Identify the elements with the following property and arrange them in increasing order of their reactivity (a). Metallic character decreases on moving from left to right in a. in the modern periodic table metallic character of the elements increases down the group and the metallic character. This table shows how the. First ionization energy, second ionization energy as well. each atom’s size is relative to the largest element, cesium. ionization energy chart of all the elements is given below.
Li V Na K Rb Cs Fr Be Mg Ca Sr Ba Ra Sc Y La
Mg Ca K Ge Ga Metallic character decreases on moving from left to right in a. This table shows how the. mg, ca, k, ge, ga 40. Atom size values are calculated from atomic radius data. calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. Metallic character decreases on moving from left to right in a. First ionization energy, second ionization energy as well. K > c a > m g > g a: in the modern periodic table metallic character of the elements increases down the group and the metallic character. Identify the elements with the following property and arrange them in increasing order of their reactivity (a). ionization energy chart of all the elements is given below. each atom’s size is relative to the largest element, cesium.
From www.researchgate.net
Ca, Mg, CaP, Mg and K(Ca+Mg of Astragalus hamosus species collected Mg Ca K Ge Ga each atom’s size is relative to the largest element, cesium. Atom size values are calculated from atomic radius data. Metallic character decreases on moving from left to right in a. This table shows how the. in the modern periodic table metallic character of the elements increases down the group and the metallic character. First ionization energy, second ionization. Mg Ca K Ge Ga.
From www.researchgate.net
Percentage of K, Mg, Ca, Fe and Al extracted in each step of sequential Mg Ca K Ge Ga calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. K > c a > m g > g a: Identify the elements with the following property and arrange them in increasing order of their reactivity (a). each atom’s size is relative to the largest element, cesium. mg,. Mg Ca K Ge Ga.
From studylib.net
H He Li Be BCNOF Ne Na Mg Al Si PS Cl Ar K Ca Sc Ti V Cr Mn Fe Mg Ca K Ge Ga First ionization energy, second ionization energy as well. each atom’s size is relative to the largest element, cesium. Atom size values are calculated from atomic radius data. ionization energy chart of all the elements is given below. mg, ca, k, ge, ga 40. This table shows how the. calcium will be more metallic than magnesium because. Mg Ca K Ge Ga.
From internetaptieka.lv
Bio Ca+K+Mg+Zn ar vitamīnu D3 un Vilkābele, 14 paciņas Mg Ca K Ge Ga This table shows how the. each atom’s size is relative to the largest element, cesium. Atom size values are calculated from atomic radius data. ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well. calcium will be more metallic than magnesium because calcium comes in period 4 of group. Mg Ca K Ge Ga.
From www.doubtnut.com
Arrange the following elements in the increasing order of their metall Mg Ca K Ge Ga in the modern periodic table metallic character of the elements increases down the group and the metallic character. This table shows how the. mg, ca, k, ge, ga 40. each atom’s size is relative to the largest element, cesium. Atom size values are calculated from atomic radius data. Identify the elements with the following property and arrange. Mg Ca K Ge Ga.
From studylib.net
Li V Na K Rb Cs Fr Be Mg Ca Sr Ba Ra Sc Y La Mg Ca K Ge Ga This table shows how the. First ionization energy, second ionization energy as well. mg, ca, k, ge, ga 40. ionization energy chart of all the elements is given below. calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. in the modern periodic table metallic character of. Mg Ca K Ge Ga.
From znanija.com
Расположите в порядке усиления металлических свойств следующие элементы Mg Ca K Ge Ga ionization energy chart of all the elements is given below. mg, ca, k, ge, ga 40. in the modern periodic table metallic character of the elements increases down the group and the metallic character. Metallic character decreases on moving from left to right in a. First ionization energy, second ionization energy as well. K > c a. Mg Ca K Ge Ga.
From www.researchgate.net
Phase diagram of the CaMg system [18] with the transition temperatures Mg Ca K Ge Ga This table shows how the. in the modern periodic table metallic character of the elements increases down the group and the metallic character. calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. K > c a > m g > g a: Metallic character decreases on moving from. Mg Ca K Ge Ga.
From www.apotheka.ee
BIO CA+K+MG+ZN+VITAMIIN D3 PULBER N28 Mg Ca K Ge Ga calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. mg, ca, k, ge, ga 40. This table shows how the. K > c a > m g > g a: each atom’s size is relative to the largest element, cesium. First ionization energy, second ionization energy as. Mg Ca K Ge Ga.
From www.youtube.com
Arrange the following elements in the increasing order of their Mg Ca K Ge Ga calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. Identify the elements with the following property and arrange them in increasing order of their reactivity (a). ionization energy chart of all the elements is given below. This table shows how the. mg, ca, k, ge, ga 40.. Mg Ca K Ge Ga.
From www.researchgate.net
Na, K, Mg, Ca, Fe, Mn, Cu and Zn content in the plots 1st Sample 2nd Mg Ca K Ge Ga First ionization energy, second ionization energy as well. This table shows how the. Atom size values are calculated from atomic radius data. calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. Identify the elements with the following property and arrange them in increasing order of their reactivity (a). . Mg Ca K Ge Ga.
From www.doubtnut.com
Density order in Be,Mg,Ca,Sr,Ba,Ra Mg Ca K Ge Ga Identify the elements with the following property and arrange them in increasing order of their reactivity (a). mg, ca, k, ge, ga 40. K > c a > m g > g a: calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. Atom size values are calculated from. Mg Ca K Ge Ga.
From www.youtube.com
Electrolyte Imbalances (Na, Ca, K, Mg) MedicalSurgical Mg Ca K Ge Ga K > c a > m g > g a: calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. This table shows how the. mg, ca, k, ge, ga 40. Atom size values are calculated from atomic radius data. Metallic character decreases on moving from left to right. Mg Ca K Ge Ga.
From www.researchgate.net
Ternary diagram illustrating the molar proportions of Mg, Ca, and Na Mg Ca K Ge Ga This table shows how the. Atom size values are calculated from atomic radius data. in the modern periodic table metallic character of the elements increases down the group and the metallic character. Identify the elements with the following property and arrange them in increasing order of their reactivity (a). First ionization energy, second ionization energy as well. mg,. Mg Ca K Ge Ga.
From www.himikatus.ru
Диаграмма состояния системы GaMg Mg Ca K Ge Ga This table shows how the. Metallic character decreases on moving from left to right in a. Atom size values are calculated from atomic radius data. mg, ca, k, ge, ga 40. ionization energy chart of all the elements is given below. K > c a > m g > g a: calcium will be more metallic than. Mg Ca K Ge Ga.
From www.manovaistine.lt
Bio CA + K + Mg + Zn su vitaminu D3, 28 pak. “MANO Mg Ca K Ge Ga K > c a > m g > g a: ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well. Identify the elements with the following property and arrange them in increasing order of their reactivity (a). mg, ca, k, ge, ga 40. in the modern periodic table metallic. Mg Ca K Ge Ga.
From www.brin-d-herbe.fr
Calcium Ca Magnesium Mg et Potassium K Mg Ca K Ge Ga in the modern periodic table metallic character of the elements increases down the group and the metallic character. This table shows how the. Identify the elements with the following property and arrange them in increasing order of their reactivity (a). Atom size values are calculated from atomic radius data. Metallic character decreases on moving from left to right in. Mg Ca K Ge Ga.
From www.studocu.com
Tabla periodica H Li Na K Rb Cs Fr Be Mg Ca Sr Ba Ra Sc Y Zr Hf Rf Ti Mg Ca K Ge Ga Identify the elements with the following property and arrange them in increasing order of their reactivity (a). ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well. calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. in the modern. Mg Ca K Ge Ga.
From www.researchgate.net
(a) Calculated temperaturedependent Mg/Ca ratio based on the applied Mg Ca K Ge Ga Metallic character decreases on moving from left to right in a. each atom’s size is relative to the largest element, cesium. calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. mg, ca, k, ge, ga 40. K > c a > m g > g a: . Mg Ca K Ge Ga.
From www.researchgate.net
Selectivity curves for KCa,Mg,Zn (figure 1a) and CaMg, CaZn, and Mg Ca K Ge Ga Identify the elements with the following property and arrange them in increasing order of their reactivity (a). ionization energy chart of all the elements is given below. Metallic character decreases on moving from left to right in a. First ionization energy, second ionization energy as well. calcium will be more metallic than magnesium because calcium comes in period. Mg Ca K Ge Ga.
From www.researchgate.net
Ternary diagram showing mole percentage of Ca, Mg, and Na + K in Mg Ca K Ge Ga each atom’s size is relative to the largest element, cesium. Atom size values are calculated from atomic radius data. calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. K > c a > m g > g a: in the modern periodic table metallic character of the. Mg Ca K Ge Ga.
From www.be-life.eu
Ca Mg K BeLife Mg Ca K Ge Ga ionization energy chart of all the elements is given below. Metallic character decreases on moving from left to right in a. in the modern periodic table metallic character of the elements increases down the group and the metallic character. First ionization energy, second ionization energy as well. K > c a > m g > g a: . Mg Ca K Ge Ga.
From www.alamy.es
Conjunto de complejo de gotas minerales. Mineral K, Cl, Ca, Cu, Mn, Na Mg Ca K Ge Ga each atom’s size is relative to the largest element, cesium. K > c a > m g > g a: ionization energy chart of all the elements is given below. in the modern periodic table metallic character of the elements increases down the group and the metallic character. Atom size values are calculated from atomic radius data.. Mg Ca K Ge Ga.
From computherm.com
MgGa CompuTherm Mg Ca K Ge Ga Metallic character decreases on moving from left to right in a. This table shows how the. in the modern periodic table metallic character of the elements increases down the group and the metallic character. Atom size values are calculated from atomic radius data. calcium will be more metallic than magnesium because calcium comes in period 4 of group. Mg Ca K Ge Ga.
From www.researchgate.net
The phase diagram of MgGa system. Download Scientific Diagram Mg Ca K Ge Ga calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. in the modern periodic table metallic character of the elements increases down the group and the metallic character. each atom’s size is relative to the largest element, cesium. ionization energy chart of all the elements is given. Mg Ca K Ge Ga.
From www.chegg.com
Solved 10. Given the MgCa phase diagram Alomic Percent Mg Ca K Ge Ga ionization energy chart of all the elements is given below. This table shows how the. in the modern periodic table metallic character of the elements increases down the group and the metallic character. mg, ca, k, ge, ga 40. K > c a > m g > g a: calcium will be more metallic than magnesium. Mg Ca K Ge Ga.
From studylib.es
H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Mg Ca K Ge Ga Identify the elements with the following property and arrange them in increasing order of their reactivity (a). This table shows how the. Atom size values are calculated from atomic radius data. in the modern periodic table metallic character of the elements increases down the group and the metallic character. calcium will be more metallic than magnesium because calcium. Mg Ca K Ge Ga.
From www.researchgate.net
a) Plot of Al + Fe versus (Mg + Ca + Ba + Sr + Na + K) for Mg Ca K Ge Ga mg, ca, k, ge, ga 40. First ionization energy, second ionization energy as well. Metallic character decreases on moving from left to right in a. Identify the elements with the following property and arrange them in increasing order of their reactivity (a). This table shows how the. each atom’s size is relative to the largest element, cesium. . Mg Ca K Ge Ga.
From www.researchgate.net
Limit and minimum required Ca and Mg contents and water hardness (Ca Mg Ca K Ge Ga Metallic character decreases on moving from left to right in a. Atom size values are calculated from atomic radius data. calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. mg, ca, k, ge, ga 40. Identify the elements with the following property and arrange them in increasing order. Mg Ca K Ge Ga.
From www.researchgate.net
The concentration of Mg, Ca, and K of the UTA, their concentration in Mg Ca K Ge Ga in the modern periodic table metallic character of the elements increases down the group and the metallic character. First ionization energy, second ionization energy as well. This table shows how the. K > c a > m g > g a: ionization energy chart of all the elements is given below. calcium will be more metallic than. Mg Ca K Ge Ga.
From e-menessaptieka.lv
BIOFARMACIJA Bio CA+K+MG+ZN ar D3 vit. pulveris, 28 gab. Piegāde visā Mg Ca K Ge Ga mg, ca, k, ge, ga 40. each atom’s size is relative to the largest element, cesium. First ionization energy, second ionization energy as well. Atom size values are calculated from atomic radius data. calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. ionization energy chart of. Mg Ca K Ge Ga.
From www.medpex.de
VITAMIN D3 K2 Hevert plus Ca Mg 1.000 I.E./2 Kaps. 60 Stück online Mg Ca K Ge Ga ionization energy chart of all the elements is given below. calcium will be more metallic than magnesium because calcium comes in period 4 of group 4 and magnesium comes. in the modern periodic table metallic character of the elements increases down the group and the metallic character. Metallic character decreases on moving from left to right in. Mg Ca K Ge Ga.
From www.reddit.com
H He Li Be B C N O F Ne Na Mg Al Si P S Cl K Ar Ca Sc Ti V Cr Mn Fe Ni Mg Ca K Ge Ga each atom’s size is relative to the largest element, cesium. Atom size values are calculated from atomic radius data. Identify the elements with the following property and arrange them in increasing order of their reactivity (a). K > c a > m g > g a: in the modern periodic table metallic character of the elements increases down. Mg Ca K Ge Ga.
From www.researchgate.net
Amounts of total K, Mg, Ca, Mn, Fe, Zn, Cu, Ni, Cd and Pb of wild Mg Ca K Ge Ga Identify the elements with the following property and arrange them in increasing order of their reactivity (a). This table shows how the. in the modern periodic table metallic character of the elements increases down the group and the metallic character. First ionization energy, second ionization energy as well. K > c a > m g > g a: . Mg Ca K Ge Ga.
From www.researchgate.net
CI, Na, Mg, Ca, and K content in I vac, vacuole; Hoag., Hoagland's Mg Ca K Ge Ga Identify the elements with the following property and arrange them in increasing order of their reactivity (a). First ionization energy, second ionization energy as well. mg, ca, k, ge, ga 40. each atom’s size is relative to the largest element, cesium. K > c a > m g > g a: in the modern periodic table metallic. Mg Ca K Ge Ga.