Calorimeter Percent Error . For example, when an exothermic reaction occurs in solution in a calorimeter, the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. For example, when an exothermic. Calorimeter is that it be well insulated, so that there is no heat lost to the surroundings during the measurement. After the calorimeter calibration, the chemist will already have a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If this condition is met, then all. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Use your calculated average deviation and relative error in your discussion. (an average deviation of 0.02 j/g°c and a relative. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. Apply the first law of thermodynamics to calorimetry.
from www.learnable.education
The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. (an average deviation of 0.02 j/g°c and a relative. Calorimeter is that it be well insulated, so that there is no heat lost to the surroundings during the measurement. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Use your calculated average deviation and relative error in your discussion. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. For example, when an exothermic reaction occurs in solution in a calorimeter, the.
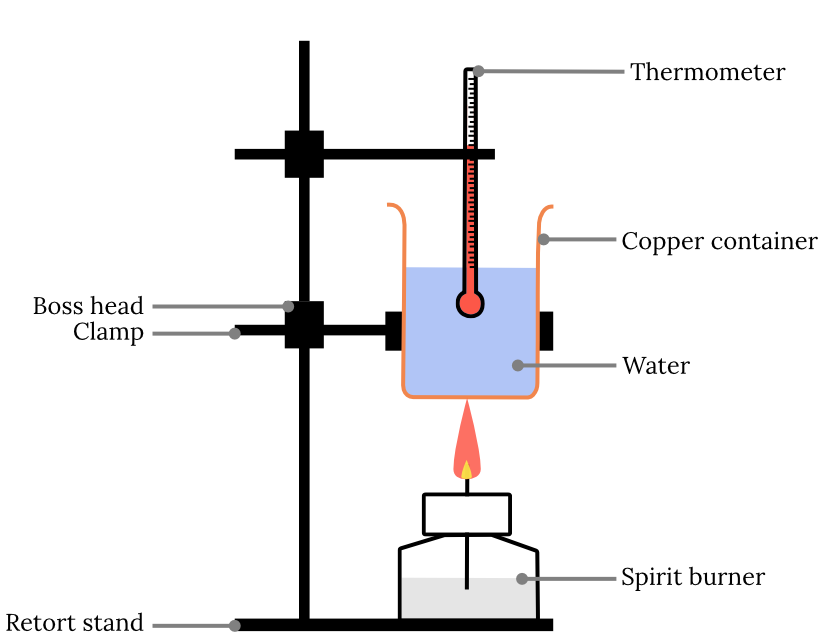
Year 11 Chemistry Practical Investigation Calorimetry Experiment
Calorimeter Percent Error A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Use your calculated average deviation and relative error in your discussion. Calorimeter is that it be well insulated, so that there is no heat lost to the surroundings during the measurement. (an average deviation of 0.02 j/g°c and a relative. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. If this condition is met, then all. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. After the calorimeter calibration, the chemist will already have a.
From tukioka-clinic.com
😂 Soda can calorimeter sources of error. What Is a Calorimeter & What Calorimeter Percent Error (an average deviation of 0.02 j/g°c and a relative. If this condition is met, then all. Use your calculated average deviation and relative error in your discussion. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. After the calorimeter calibration, the chemist will already have. Calorimeter Percent Error.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Percent Error Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter is that it be well insulated, so that there is no. Calorimeter Percent Error.
From hxelhkhvw.blob.core.windows.net
How To Calculate Percent Error In Calorimetry at Smart blog Calorimeter Percent Error (an average deviation of 0.02 j/g°c and a relative. After the calorimeter calibration, the chemist will already have a. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the. Calorimeter Percent Error.
From www.scribd.com
Error calorimeter constant PDF Temperature Heat Calorimeter Percent Error The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. After the calorimeter calibration, the chemist will already have a. If this condition is met, then all. (an average deviation of 0.02 j/g°c and a relative. Basically, a calorimeter. Calorimeter Percent Error.
From www.slideshare.net
Calculating percent error Calorimeter Percent Error Calorimeter is that it be well insulated, so that there is no heat lost to the surroundings during the measurement. If this condition is met, then all. After the calorimeter calibration, the chemist will already have a. Apply the first law of thermodynamics to calorimetry. Use your calculated average deviation and relative error in your discussion. For example, when an. Calorimeter Percent Error.
From hxelhkhvw.blob.core.windows.net
How To Calculate Percent Error In Calorimetry at Smart blog Calorimeter Percent Error The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. If this condition is met, then all. Use your calculated average deviation and relative error in your discussion. For example, when an exothermic reaction occurs in solution in a. Calorimeter Percent Error.
From www.numerade.com
SOLVED EXPERIMENT Calorimetry REPORT FORM Date 11/18/2021 Mass of Calorimeter Percent Error Calorimeter is that it be well insulated, so that there is no heat lost to the surroundings during the measurement. The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. Use your calculated average deviation and relative error in. Calorimeter Percent Error.
From www.numerade.com
SOLVEDDATA Table I First metal Cwater JIg ) Csubstance JI(g C Calorimeter Percent Error Apply the first law of thermodynamics to calorimetry. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. If this condition is met, then all. (an average deviation of 0.02 j/g°c and a relative. For example, when an exothermic. The information presented here is intended to give parr calorimeter users an overview of the basic principals. Calorimeter Percent Error.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimeter Percent Error If this condition is met, then all. (an average deviation of 0.02 j/g°c and a relative. Use your calculated average deviation and relative error in your discussion. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a. Calorimeter Percent Error.
From www.chegg.com
Solved What percent error does he neglect of the calorimeter Calorimeter Percent Error Apply the first law of thermodynamics to calorimetry. The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. (an average deviation of 0.02 j/g°c and a relative. After the calorimeter calibration, the chemist will already have a. Calorimeter is. Calorimeter Percent Error.
From www.researchgate.net
Actual and Forecast Values of the First Flux Calorimeter Readings (Heat Calorimeter Percent Error For example, when an exothermic. Use your calculated average deviation and relative error in your discussion. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. For example, when an exothermic reaction occurs in solution in a calorimeter, the. The information presented here is intended to give parr calorimeter users an overview of the basic. Calorimeter Percent Error.
From www.numerade.com
SOLVED For the specific heat of water, use calorie/gram. With the data Calorimeter Percent Error A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. After the calorimeter calibration, the chemist will already have a. If this condition is met, then all. Use your calculated average deviation and relative error in your discussion. Basically, a calorimeter measures the change in temperature. Calorimeter Percent Error.
From www.numerade.com
SOLVED In the bomb calorimeter experiment, if the theoretical value of Calorimeter Percent Error If this condition is met, then all. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Apply the first law of thermodynamics to. Calorimeter Percent Error.
From github.com
GitHub saviss33/Calorimeter Temperature error associated in heat Calorimeter Percent Error For example, when an exothermic reaction occurs in solution in a calorimeter, the. Apply the first law of thermodynamics to calorimetry. The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. A calorimeter is a device used to measure. Calorimeter Percent Error.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Calorimeter Percent Error Use your calculated average deviation and relative error in your discussion. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. After the. Calorimeter Percent Error.
From www.youtube.com
Calorimetry calculation YouTube Calorimeter Percent Error For example, when an exothermic. After the calorimeter calibration, the chemist will already have a. Use your calculated average deviation and relative error in your discussion. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimeter is that it be well insulated, so that there is no heat lost to the surroundings during the measurement. Apply. Calorimeter Percent Error.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimeter Percent Error Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. If this condition is met, then all. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The information presented here is intended to give parr calorimeter users an overview of. Calorimeter Percent Error.
From www.chegg.com
or this part of the lab, you will analyze recorded Calorimeter Percent Error The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. For example, when an exothermic. (an average deviation of 0.02 j/g°c and a relative. If this condition is met, then all. A calorimeter is a device used to measure. Calorimeter Percent Error.
From www.slideserve.com
PPT Formation Reactions PowerPoint Presentation ID2308925 Calorimeter Percent Error Apply the first law of thermodynamics to calorimetry. If this condition is met, then all. For example, when an exothermic. After the calorimeter calibration, the chemist will already have a. Calorimeter is that it be well insulated, so that there is no heat lost to the surroundings during the measurement. A calorimeter is a device used to measure the amount. Calorimeter Percent Error.
From mathsathome.com
How to Calculate the Percentage Error (Pictures and Examples Calorimeter Percent Error Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. If this condition is met, then all. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Calorimeter is that it be well insulated, so that there is no heat lost to the surroundings during the measurement. For example, when an. Calorimeter Percent Error.
From www.cuemath.com
How to Calculate Percent Error? Concept and Calculation, Meaning Calorimeter Percent Error For example, when an exothermic. The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. (an average deviation of 0.02 j/g°c and a relative. If this condition is met, then all. A calorimeter is a device used to measure. Calorimeter Percent Error.
From www.tffn.net
Solving Calorimetry Problems A StepbyStep Guide The Enlightened Calorimeter Percent Error For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimeter is that it be well insulated, so that there is no heat lost to the surroundings during the measurement. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If this condition. Calorimeter Percent Error.
From www.slideserve.com
PPT Energy in food PowerPoint Presentation, free download ID5830775 Calorimeter Percent Error Use your calculated average deviation and relative error in your discussion. The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. (an average deviation of 0.02 j/g°c and a relative. A calorimeter is a device used to measure the. Calorimeter Percent Error.
From users.highland.edu
Calorimetry Calorimeter Percent Error For example, when an exothermic reaction occurs in solution in a calorimeter, the. The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply. Calorimeter Percent Error.
From hxelhkhvw.blob.core.windows.net
How To Calculate Percent Error In Calorimetry at Smart blog Calorimeter Percent Error Apply the first law of thermodynamics to calorimetry. Calorimeter is that it be well insulated, so that there is no heat lost to the surroundings during the measurement. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the.. Calorimeter Percent Error.
From www.chegg.com
Solved A student transfers a heated sample of pure iron Calorimeter Percent Error After the calorimeter calibration, the chemist will already have a. Apply the first law of thermodynamics to calorimetry. The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. If this condition is met, then all. For example, when an. Calorimeter Percent Error.
From www.researchgate.net
Types of calorimeters with distinguished marks and problems Download Calorimeter Percent Error Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. (an average deviation of 0.02 j/g°c and a relative. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Calorimeter Percent Error.
From www.numerade.com
SOLVED Hot water of temperature (T ) is added to the calorimeter and Calorimeter Percent Error The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount. Calorimeter Percent Error.
From www.numerade.com
Post Simulation Questions (1) Mass of iron [Initial temperature of Fe Calorimeter Percent Error The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. Use your calculated average deviation and relative error in your discussion. (an average deviation of 0.02 j/g°c and a relative. Basically, a calorimeter measures the change in temperature of. Calorimeter Percent Error.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimeter Percent Error Calorimeter is that it be well insulated, so that there is no heat lost to the surroundings during the measurement. After the calorimeter calibration, the chemist will already have a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal. Calorimeter Percent Error.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimeter Percent Error Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. For example, when an exothermic. Use your calculated average deviation and relative error in your discussion. (an average deviation of 0.02 j/g°c and a relative. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat. Calorimeter Percent Error.
From www.numerade.com
SOLVED 9. for TNT can be calculated using 𝛥𝐻𝑐𝑜𝑚𝑏 = 𝐾𝑐𝑎𝑙(𝛥𝑇)⁄𝑛 Calorimeter Percent Error After the calorimeter calibration, the chemist will already have a. (an average deviation of 0.02 j/g°c and a relative. Apply the first law of thermodynamics to calorimetry. The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. Calorimeter is. Calorimeter Percent Error.
From hxelhkhvw.blob.core.windows.net
How To Calculate Percent Error In Calorimetry at Smart blog Calorimeter Percent Error Calorimeter is that it be well insulated, so that there is no heat lost to the surroundings during the measurement. (an average deviation of 0.02 j/g°c and a relative. After the calorimeter calibration, the chemist will already have a. Apply the first law of thermodynamics to calorimetry. Basically, a calorimeter measures the change in temperature of the calorimeter and its. Calorimeter Percent Error.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimeter Percent Error The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot. Calorimeter Percent Error.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6655927 Calorimeter Percent Error The information presented here is intended to give parr calorimeter users an overview of the basic principals involved in measuring the heat of combustion (calorific value) of solid and liquid fuels,. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic. Calorimeter Percent Error.