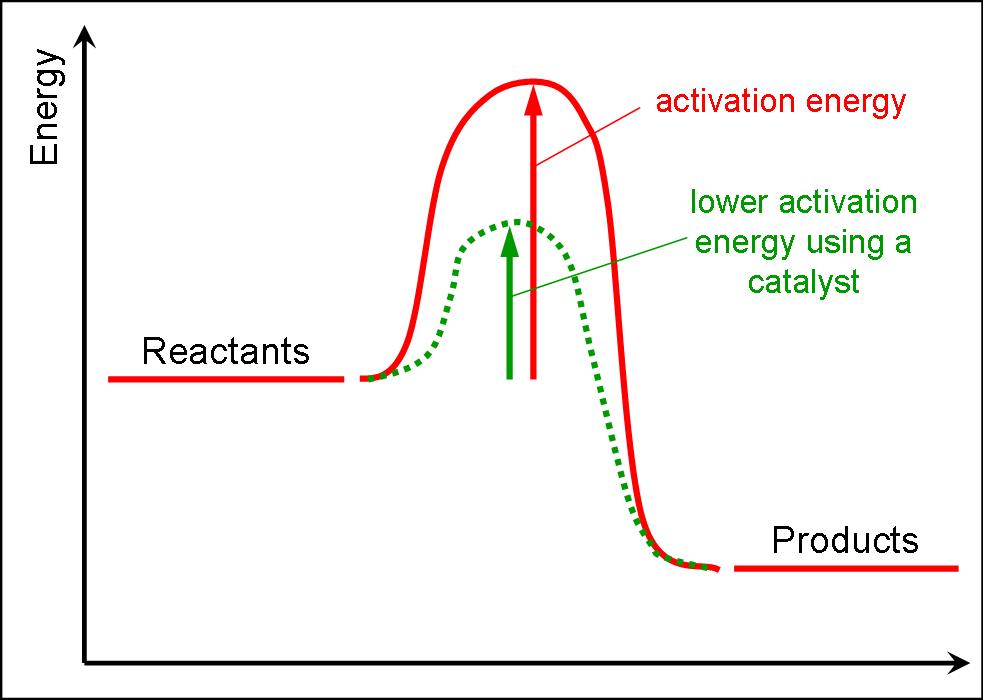
Does Catalyst Increase Activation Energy . learn how adding a catalyst can increase the rate of a reaction by lowering the activation energy. the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. learn how activation energy is the minimum energy barrier that molecules must overcome to react, and how. See examples, diagrams and explanations of the. learn how catalysts speed up reactions by providing an alternative route with lower activation energy.
from as-bio-and-chem.blogspot.com
the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. See examples, diagrams and explanations of the. learn how activation energy is the minimum energy barrier that molecules must overcome to react, and how. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how adding a catalyst can increase the rate of a reaction by lowering the activation energy.
Bio+Chem Notes. ^^ Recapping Rates of Reaction
Does Catalyst Increase Activation Energy learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how activation energy is the minimum energy barrier that molecules must overcome to react, and how. learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. See examples, diagrams and explanations of the. learn how adding a catalyst can increase the rate of a reaction by lowering the activation energy. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Does Catalyst Increase Activation Energy learn how adding a catalyst can increase the rate of a reaction by lowering the activation energy. the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. learn how activation energy is the minimum energy barrier that molecules must overcome to react, and how. . Does Catalyst Increase Activation Energy.
From fyoefmgmv.blob.core.windows.net
Do Catalysts Increase Reaction Rate at Stephen Johnson blog Does Catalyst Increase Activation Energy learn how activation energy is the minimum energy barrier that molecules must overcome to react, and how. learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. learn how. Does Catalyst Increase Activation Energy.
From www.slideserve.com
PPT Reaction PowerPoint Presentation ID1835084 Does Catalyst Increase Activation Energy catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. learn how adding a catalyst can increase the rate of a reaction by lowering the activation energy. the effect. Does Catalyst Increase Activation Energy.
From dragonbeeotch4sschematic.z21.web.core.windows.net
Energy Diagram For Chemical Reaction Does Catalyst Increase Activation Energy learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how adding a catalyst can increase the rate of a reaction by lowering the activation energy. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. See examples, diagrams and. Does Catalyst Increase Activation Energy.
From 2012books.lardbucket.org
Catalysis Does Catalyst Increase Activation Energy the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. learn how adding a catalyst can increase the rate of a reaction by lowering the activation energy. learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. learn. Does Catalyst Increase Activation Energy.
From guidepartrefractor.z21.web.core.windows.net
Energy Diagram Catalyzed Reaction Does Catalyst Increase Activation Energy learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. learn how adding a catalyst can increase the rate of a reaction by lowering the activation energy. learn how. Does Catalyst Increase Activation Energy.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Does Catalyst Increase Activation Energy See examples, diagrams and explanations of the. learn how adding a catalyst can increase the rate of a reaction by lowering the activation energy. the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. learn how catalysts speed up reactions by providing an alternative route. Does Catalyst Increase Activation Energy.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Does Catalyst Increase Activation Energy learn how catalysts speed up reactions by providing an alternative route with lower activation energy. the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. See examples, diagrams and. Does Catalyst Increase Activation Energy.
From www.workmanagementsolutions.com.au
An Analogy The Activation Energy and Catalysis in Business Operations Does Catalyst Increase Activation Energy See examples, diagrams and explanations of the. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. the effect of a catalyst on the activation energy is shown on a chart. Does Catalyst Increase Activation Energy.
From www.sliderbase.com
Catalysis Presentation Chemistry Does Catalyst Increase Activation Energy catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. See examples, diagrams and explanations of the. learn how activation energy is the minimum energy barrier that molecules must overcome to react, and how. learn how adding a catalyst can increase the rate of a reaction. Does Catalyst Increase Activation Energy.
From gioaralqv.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Reaction In Such A Manner Does Catalyst Increase Activation Energy the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. learn how activation energy is the minimum energy barrier that molecules must overcome to react, and how. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as. Does Catalyst Increase Activation Energy.
From slideplayer.com
A catalyst lowers activation energy. ppt download Does Catalyst Increase Activation Energy See examples, diagrams and explanations of the. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close. Does Catalyst Increase Activation Energy.
From www.chim.lu
Activation energy and catalysis Does Catalyst Increase Activation Energy learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. See examples, diagrams and explanations of the. learn how catalysts speed up reactions by providing an alternative route with. Does Catalyst Increase Activation Energy.
From www.tes.com
Catalysts and Activation Energy GCSE lesson (SC18c CC14c) Teaching Does Catalyst Increase Activation Energy See examples, diagrams and explanations of the. learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. learn how activation energy is the minimum energy barrier that molecules must overcome to react, and how. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy,. Does Catalyst Increase Activation Energy.
From vdocuments.mx
Catalysts Catalystlowers activation energy of a chemical reaction and Does Catalyst Increase Activation Energy learn how adding a catalyst can increase the rate of a reaction by lowering the activation energy. See examples, diagrams and explanations of the. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. learn how catalysts lower the activation energy of reactions by providing a. Does Catalyst Increase Activation Energy.
From www.chemistrystudent.com
Boltzmann Distribution Curves (ALevel) ChemistryStudent Does Catalyst Increase Activation Energy the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. See examples, diagrams and explanations of the. learn how activation energy is the minimum. Does Catalyst Increase Activation Energy.
From www.numerade.com
SOLVEDHow does a catalyst that increases reaction rate lower the Does Catalyst Increase Activation Energy the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. learn how adding a catalyst can increase the rate of a reaction by lowering. Does Catalyst Increase Activation Energy.
From slideplayer.com
Chapter 24 Chemical Reactions and Enzymes. ppt download Does Catalyst Increase Activation Energy learn how catalysts speed up reactions by providing an alternative route with lower activation energy. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile. Does Catalyst Increase Activation Energy.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Does Catalyst Increase Activation Energy catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. learn how adding a catalyst can increase the rate of a reaction by lowering the activation energy. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. See examples, diagrams and. Does Catalyst Increase Activation Energy.
From byjus.com
How does catalyst affect activation energy? Does Catalyst Increase Activation Energy learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. catalysts increase the. Does Catalyst Increase Activation Energy.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Does Catalyst Increase Activation Energy learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how adding a catalyst can increase the rate of a reaction by lowering the activation energy. learn how activation energy is the minimum energy. Does Catalyst Increase Activation Energy.
From slideplayer.com
Enzymes. ppt download Does Catalyst Increase Activation Energy the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how activation energy is the minimum energy barrier that molecules must overcome to react, and how. See examples, diagrams. Does Catalyst Increase Activation Energy.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Does Catalyst Increase Activation Energy learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. learn how adding a catalyst can increase the rate of a reaction by lowering the activation energy. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. the effect of a catalyst on the activation. Does Catalyst Increase Activation Energy.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Does Catalyst Increase Activation Energy the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. learn how adding a catalyst can increase the rate of a reaction by lowering the activation energy. learn how activation energy is the minimum energy barrier that molecules must overcome to react, and how. . Does Catalyst Increase Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Does Catalyst Increase Activation Energy learn how adding a catalyst can increase the rate of a reaction by lowering the activation energy. learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. See examples, diagrams. Does Catalyst Increase Activation Energy.
From slideplayer.com
Lecture 1405 Reaction Mechanism and Catalysis ppt download Does Catalyst Increase Activation Energy the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. learn how activation energy is the minimum energy barrier that molecules must overcome to react, and how. learn. Does Catalyst Increase Activation Energy.
From as-bio-and-chem.blogspot.com
Bio+Chem Notes. ^^ Recapping Rates of Reaction Does Catalyst Increase Activation Energy the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. learn how adding a catalyst can increase the rate of a reaction by lowering the activation energy. See examples, diagrams and explanations of the. learn how catalysts lower the activation energy of reactions by providing. Does Catalyst Increase Activation Energy.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Does Catalyst Increase Activation Energy learn how activation energy is the minimum energy barrier that molecules must overcome to react, and how. See examples, diagrams and explanations of the. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. learn how catalysts lower the activation energy of reactions by providing a. Does Catalyst Increase Activation Energy.
From exyfvrfps.blob.core.windows.net
Do All Catalysts Lower Activation Energy at Shirley Tolliver blog Does Catalyst Increase Activation Energy learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. learn how activation energy is the minimum energy barrier that molecules must overcome to react, and how. learn how adding a catalyst can increase the rate of a reaction by lowering the activation energy. catalysts increase the rates of reactions. Does Catalyst Increase Activation Energy.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Does Catalyst Increase Activation Energy learn how activation energy is the minimum energy barrier that molecules must overcome to react, and how. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. catalysts increase. Does Catalyst Increase Activation Energy.
From www.thoughtco.com
Catalysis Definition in Chemistry Does Catalyst Increase Activation Energy learn how activation energy is the minimum energy barrier that molecules must overcome to react, and how. See examples, diagrams and explanations of the. catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the. learn how adding a catalyst can increase the rate of a reaction. Does Catalyst Increase Activation Energy.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Does Catalyst Increase Activation Energy learn how catalysts speed up reactions by providing an alternative route with lower activation energy. See examples, diagrams and explanations of the. the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. catalysts increase the rates of reactions by providing a new mechanism that has. Does Catalyst Increase Activation Energy.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free Does Catalyst Increase Activation Energy the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. learn how activation energy is the minimum energy barrier that molecules must overcome to react, and how. catalysts. Does Catalyst Increase Activation Energy.
From gioyfzlvn.blob.core.windows.net
Which Catalysts Lower Activation Energy at Ronald Lerner blog Does Catalyst Increase Activation Energy learn how catalysts lower the activation energy of reactions by providing a pathway or transition state. See examples, diagrams and explanations of the. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close. Does Catalyst Increase Activation Energy.
From slideplayer.com
Biochemistry 412 Enzyme I March 29th, ppt download Does Catalyst Increase Activation Energy the effect of a catalyst on the activation energy is shown on a chart called a reaction profile close reaction profile chart. See examples, diagrams and explanations of the. learn how activation energy is the minimum energy barrier that molecules must overcome to react, and how. catalysts increase the rates of reactions by providing a new mechanism. Does Catalyst Increase Activation Energy.