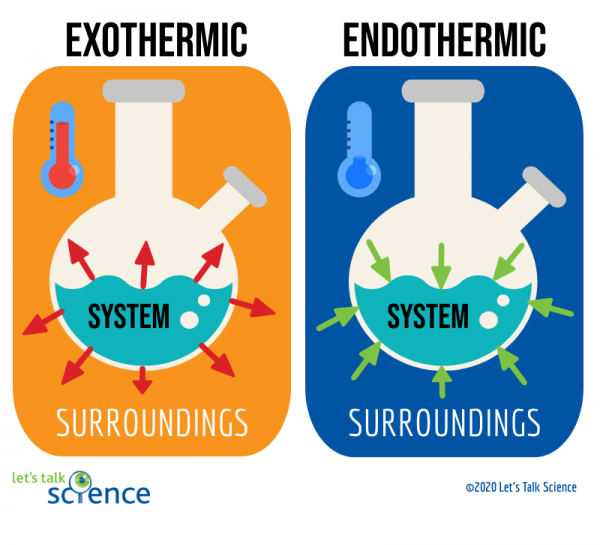
Hot Pack Cold Pack Exothermic And Endothermic Processes . When the temperature decreases, as. In an endothermic reaction, a substance takes heat from its surroundings. Exothermic and endothermic reactions are all around us. Allow students to feel the temperature change in an activated cold pack and an activated hot pack. Explore endothermic and exothermic process by making a cold pack or hot pack. Students design and build a reusable heat pack. Tell students that they will explore how. Ever hurt yourself during a game or on the playground and need a. When the hot pack is activated by squeezing it, a small amount of water is mixed with the sodium acetate, which triggers an exothermic. The sodium thiosulfate needs energy to dissolve, so. How do hot packs and cold packs change temperature? Tell students that scientists describe temperature changes that occur when substances interact as either endothermic or exothermic. They are required to determine what chemical reaction generates heat and is reusable.
from letstalkscience.ca
Students design and build a reusable heat pack. Explore endothermic and exothermic process by making a cold pack or hot pack. How do hot packs and cold packs change temperature? They are required to determine what chemical reaction generates heat and is reusable. Tell students that they will explore how. When the temperature decreases, as. When the hot pack is activated by squeezing it, a small amount of water is mixed with the sodium acetate, which triggers an exothermic. In an endothermic reaction, a substance takes heat from its surroundings. Exothermic and endothermic reactions are all around us. The sodium thiosulfate needs energy to dissolve, so.
The Cold Pack A Chilly Example of an Endothermic Reaction Let's Talk Science
Hot Pack Cold Pack Exothermic And Endothermic Processes Exothermic and endothermic reactions are all around us. When the hot pack is activated by squeezing it, a small amount of water is mixed with the sodium acetate, which triggers an exothermic. They are required to determine what chemical reaction generates heat and is reusable. Exothermic and endothermic reactions are all around us. The sodium thiosulfate needs energy to dissolve, so. Explore endothermic and exothermic process by making a cold pack or hot pack. Allow students to feel the temperature change in an activated cold pack and an activated hot pack. In an endothermic reaction, a substance takes heat from its surroundings. How do hot packs and cold packs change temperature? Ever hurt yourself during a game or on the playground and need a. When the temperature decreases, as. Tell students that scientists describe temperature changes that occur when substances interact as either endothermic or exothermic. Tell students that they will explore how. Students design and build a reusable heat pack.
From www.pinterest.co.uk
Cold Pack Chemistry Exploring Endothermic and Exothermic Reactions Lesson Plan Stem lesson Hot Pack Cold Pack Exothermic And Endothermic Processes When the temperature decreases, as. How do hot packs and cold packs change temperature? Allow students to feel the temperature change in an activated cold pack and an activated hot pack. When the hot pack is activated by squeezing it, a small amount of water is mixed with the sodium acetate, which triggers an exothermic. Ever hurt yourself during a. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From mylaldnorman.blogspot.com
Cold Pack Endothermic or Exothermic MylaldNorman Hot Pack Cold Pack Exothermic And Endothermic Processes Exothermic and endothermic reactions are all around us. In an endothermic reaction, a substance takes heat from its surroundings. Ever hurt yourself during a game or on the playground and need a. The sodium thiosulfate needs energy to dissolve, so. Explore endothermic and exothermic process by making a cold pack or hot pack. When the temperature decreases, as. Allow students. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From www.slideserve.com
PPT Chapter 7 Hot & Cold Packs PowerPoint Presentation, free download ID2507233 Hot Pack Cold Pack Exothermic And Endothermic Processes When the hot pack is activated by squeezing it, a small amount of water is mixed with the sodium acetate, which triggers an exothermic. Ever hurt yourself during a game or on the playground and need a. Tell students that scientists describe temperature changes that occur when substances interact as either endothermic or exothermic. Explore endothermic and exothermic process by. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From online-learning-college.com
Exothermic and endothermic reactions Key differences Hot Pack Cold Pack Exothermic And Endothermic Processes Explore endothermic and exothermic process by making a cold pack or hot pack. How do hot packs and cold packs change temperature? Students design and build a reusable heat pack. The sodium thiosulfate needs energy to dissolve, so. Tell students that scientists describe temperature changes that occur when substances interact as either endothermic or exothermic. When the hot pack is. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From brooklynewtortega.blogspot.com
Cold Pack Endothermic or Exothermic BrooklynewtOrtega Hot Pack Cold Pack Exothermic And Endothermic Processes Exothermic and endothermic reactions are all around us. In an endothermic reaction, a substance takes heat from its surroundings. The sodium thiosulfate needs energy to dissolve, so. Ever hurt yourself during a game or on the playground and need a. Tell students that scientists describe temperature changes that occur when substances interact as either endothermic or exothermic. Tell students that. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From chhattisgarh.pscnotes.com
Exothermic and endothermic reactions CGPCS Exam Preparation Hot Pack Cold Pack Exothermic And Endothermic Processes When the temperature decreases, as. Allow students to feel the temperature change in an activated cold pack and an activated hot pack. Students design and build a reusable heat pack. Tell students that they will explore how. They are required to determine what chemical reaction generates heat and is reusable. Ever hurt yourself during a game or on the playground. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From www.slideserve.com
PPT Chapter 7 Hot & Cold Packs PowerPoint Presentation, free download ID2507233 Hot Pack Cold Pack Exothermic And Endothermic Processes When the hot pack is activated by squeezing it, a small amount of water is mixed with the sodium acetate, which triggers an exothermic. Exothermic and endothermic reactions are all around us. Allow students to feel the temperature change in an activated cold pack and an activated hot pack. Ever hurt yourself during a game or on the playground and. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation ID1277165 Hot Pack Cold Pack Exothermic And Endothermic Processes How do hot packs and cold packs change temperature? When the hot pack is activated by squeezing it, a small amount of water is mixed with the sodium acetate, which triggers an exothermic. Tell students that scientists describe temperature changes that occur when substances interact as either endothermic or exothermic. Students design and build a reusable heat pack. Exothermic and. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From www.pinterest.com
Understanding Endothermic and Exothermic Reactions Exothermic reaction, Chemistry experiments Hot Pack Cold Pack Exothermic And Endothermic Processes Exothermic and endothermic reactions are all around us. Ever hurt yourself during a game or on the playground and need a. When the temperature decreases, as. Explore endothermic and exothermic process by making a cold pack or hot pack. Tell students that scientists describe temperature changes that occur when substances interact as either endothermic or exothermic. How do hot packs. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From slideplayer.com
Endothermic and Exothermic Reactions ppt download Hot Pack Cold Pack Exothermic And Endothermic Processes Exothermic and endothermic reactions are all around us. The sodium thiosulfate needs energy to dissolve, so. Tell students that scientists describe temperature changes that occur when substances interact as either endothermic or exothermic. Students design and build a reusable heat pack. Explore endothermic and exothermic process by making a cold pack or hot pack. Ever hurt yourself during a game. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From slideplayer.com
Endothermic & Exothermic Reactions ppt download Hot Pack Cold Pack Exothermic And Endothermic Processes When the temperature decreases, as. Explore endothermic and exothermic process by making a cold pack or hot pack. How do hot packs and cold packs change temperature? In an endothermic reaction, a substance takes heat from its surroundings. When the hot pack is activated by squeezing it, a small amount of water is mixed with the sodium acetate, which triggers. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From teachingscience.us
Exothermic and Endothermic Reactions Hot Pack Cold Pack Exothermic And Endothermic Processes When the temperature decreases, as. Students design and build a reusable heat pack. How do hot packs and cold packs change temperature? Ever hurt yourself during a game or on the playground and need a. Explore endothermic and exothermic process by making a cold pack or hot pack. The sodium thiosulfate needs energy to dissolve, so. They are required to. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From www.tes.com
KS3 Chemistry Endothermic and Exothermic Reactions Hot and Cold Lesson Presentation and Hot Pack Cold Pack Exothermic And Endothermic Processes When the temperature decreases, as. Exothermic and endothermic reactions are all around us. Students design and build a reusable heat pack. The sodium thiosulfate needs energy to dissolve, so. How do hot packs and cold packs change temperature? Allow students to feel the temperature change in an activated cold pack and an activated hot pack. Tell students that scientists describe. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From sciencenotes.org
Endothermic Reactions Definition and Examples Hot Pack Cold Pack Exothermic And Endothermic Processes Students design and build a reusable heat pack. Tell students that they will explore how. Explore endothermic and exothermic process by making a cold pack or hot pack. Allow students to feel the temperature change in an activated cold pack and an activated hot pack. They are required to determine what chemical reaction generates heat and is reusable. Exothermic and. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From slideplayer.com
NOTES 9 Chemical Reactions ppt download Hot Pack Cold Pack Exothermic And Endothermic Processes When the hot pack is activated by squeezing it, a small amount of water is mixed with the sodium acetate, which triggers an exothermic. Exothermic and endothermic reactions are all around us. In an endothermic reaction, a substance takes heat from its surroundings. Ever hurt yourself during a game or on the playground and need a. Tell students that they. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From www.youtube.com
Hot/Cold Pack Endothermic and Exothermic Reactions (4/11) YouTube Hot Pack Cold Pack Exothermic And Endothermic Processes When the temperature decreases, as. Explore endothermic and exothermic process by making a cold pack or hot pack. When the hot pack is activated by squeezing it, a small amount of water is mixed with the sodium acetate, which triggers an exothermic. In an endothermic reaction, a substance takes heat from its surroundings. Tell students that they will explore how.. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation, free download ID1277165 Hot Pack Cold Pack Exothermic And Endothermic Processes Exothermic and endothermic reactions are all around us. When the hot pack is activated by squeezing it, a small amount of water is mixed with the sodium acetate, which triggers an exothermic. Ever hurt yourself during a game or on the playground and need a. Allow students to feel the temperature change in an activated cold pack and an activated. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From www.slideserve.com
PPT Chapter 7 Hot & Cold Packs PowerPoint Presentation ID2507233 Hot Pack Cold Pack Exothermic And Endothermic Processes In an endothermic reaction, a substance takes heat from its surroundings. When the temperature decreases, as. The sodium thiosulfate needs energy to dissolve, so. Tell students that they will explore how. How do hot packs and cold packs change temperature? Explore endothermic and exothermic process by making a cold pack or hot pack. Students design and build a reusable heat. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From humanbiology.pressbooks.tru.ca
3.9 Energy in Chemical Reactions Human Biology Hot Pack Cold Pack Exothermic And Endothermic Processes They are required to determine what chemical reaction generates heat and is reusable. Students design and build a reusable heat pack. Explore endothermic and exothermic process by making a cold pack or hot pack. Tell students that scientists describe temperature changes that occur when substances interact as either endothermic or exothermic. When the hot pack is activated by squeezing it,. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation, free download ID1277165 Hot Pack Cold Pack Exothermic And Endothermic Processes Tell students that they will explore how. When the temperature decreases, as. How do hot packs and cold packs change temperature? They are required to determine what chemical reaction generates heat and is reusable. Explore endothermic and exothermic process by making a cold pack or hot pack. Ever hurt yourself during a game or on the playground and need a.. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From www.thoughtco.com
Make a Cold Pack from Hot Ice Hot Pack Cold Pack Exothermic And Endothermic Processes They are required to determine what chemical reaction generates heat and is reusable. Allow students to feel the temperature change in an activated cold pack and an activated hot pack. How do hot packs and cold packs change temperature? Ever hurt yourself during a game or on the playground and need a. Explore endothermic and exothermic process by making a. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation, free download ID1277165 Hot Pack Cold Pack Exothermic And Endothermic Processes They are required to determine what chemical reaction generates heat and is reusable. Tell students that they will explore how. How do hot packs and cold packs change temperature? The sodium thiosulfate needs energy to dissolve, so. Explore endothermic and exothermic process by making a cold pack or hot pack. Students design and build a reusable heat pack. When the. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From slideplayer.com
Chapter 12 Solutions. ppt download Hot Pack Cold Pack Exothermic And Endothermic Processes In an endothermic reaction, a substance takes heat from its surroundings. Allow students to feel the temperature change in an activated cold pack and an activated hot pack. When the hot pack is activated by squeezing it, a small amount of water is mixed with the sodium acetate, which triggers an exothermic. When the temperature decreases, as. Tell students that. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From letstalkscience.ca
The Cold Pack A Chilly Example of an Endothermic Reaction Let's Talk Science Hot Pack Cold Pack Exothermic And Endothermic Processes Tell students that they will explore how. In an endothermic reaction, a substance takes heat from its surroundings. Students design and build a reusable heat pack. The sodium thiosulfate needs energy to dissolve, so. How do hot packs and cold packs change temperature? Allow students to feel the temperature change in an activated cold pack and an activated hot pack.. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation, free download ID1277165 Hot Pack Cold Pack Exothermic And Endothermic Processes Tell students that scientists describe temperature changes that occur when substances interact as either endothermic or exothermic. Tell students that they will explore how. Students design and build a reusable heat pack. Ever hurt yourself during a game or on the playground and need a. They are required to determine what chemical reaction generates heat and is reusable. In an. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From www.shutterstock.com
Endothermic Exothermic Chemical Reactions Cold Cubes Stock Vector (Royalty Free) 2000465120 Hot Pack Cold Pack Exothermic And Endothermic Processes The sodium thiosulfate needs energy to dissolve, so. In an endothermic reaction, a substance takes heat from its surroundings. When the hot pack is activated by squeezing it, a small amount of water is mixed with the sodium acetate, which triggers an exothermic. Explore endothermic and exothermic process by making a cold pack or hot pack. Tell students that they. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From www.youtube.com
Instant hot pack and instant cold pack, application of exothermic and endothermic reactions Hot Pack Cold Pack Exothermic And Endothermic Processes Exothermic and endothermic reactions are all around us. Tell students that scientists describe temperature changes that occur when substances interact as either endothermic or exothermic. When the temperature decreases, as. How do hot packs and cold packs change temperature? Students design and build a reusable heat pack. They are required to determine what chemical reaction generates heat and is reusable.. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From docslib.org
Hot Pack Cold Pack Exothermic & Endothermic Processes DocsLib Hot Pack Cold Pack Exothermic And Endothermic Processes Tell students that they will explore how. The sodium thiosulfate needs energy to dissolve, so. When the temperature decreases, as. Allow students to feel the temperature change in an activated cold pack and an activated hot pack. Students design and build a reusable heat pack. How do hot packs and cold packs change temperature? Tell students that scientists describe temperature. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From fphoto.photoshelter.com
science chemical reaction endothermic cold pack Fundamental Photographs The Art of Science Hot Pack Cold Pack Exothermic And Endothermic Processes Allow students to feel the temperature change in an activated cold pack and an activated hot pack. Tell students that they will explore how. When the temperature decreases, as. They are required to determine what chemical reaction generates heat and is reusable. How do hot packs and cold packs change temperature? The sodium thiosulfate needs energy to dissolve, so. Tell. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation, free download ID1277165 Hot Pack Cold Pack Exothermic And Endothermic Processes Explore endothermic and exothermic process by making a cold pack or hot pack. The sodium thiosulfate needs energy to dissolve, so. Ever hurt yourself during a game or on the playground and need a. Students design and build a reusable heat pack. They are required to determine what chemical reaction generates heat and is reusable. In an endothermic reaction, a. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From question.pandai.org
Endothermic and exothermic reactions Hot Pack Cold Pack Exothermic And Endothermic Processes Explore endothermic and exothermic process by making a cold pack or hot pack. The sodium thiosulfate needs energy to dissolve, so. They are required to determine what chemical reaction generates heat and is reusable. Students design and build a reusable heat pack. When the temperature decreases, as. Tell students that scientists describe temperature changes that occur when substances interact as. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From www.slideserve.com
PPT Hot Packs and Cold Packs PowerPoint Presentation, free download ID5856594 Hot Pack Cold Pack Exothermic And Endothermic Processes In an endothermic reaction, a substance takes heat from its surroundings. Students design and build a reusable heat pack. They are required to determine what chemical reaction generates heat and is reusable. Exothermic and endothermic reactions are all around us. The sodium thiosulfate needs energy to dissolve, so. Tell students that they will explore how. Explore endothermic and exothermic process. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From dokumen.tips
(PPTX) Endothermic and Exothermic Chemical Reactions Exothermic Chemical reactions that release Hot Pack Cold Pack Exothermic And Endothermic Processes Students design and build a reusable heat pack. How do hot packs and cold packs change temperature? Tell students that scientists describe temperature changes that occur when substances interact as either endothermic or exothermic. Ever hurt yourself during a game or on the playground and need a. The sodium thiosulfate needs energy to dissolve, so. When the hot pack is. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From www.numerade.com
SOLVED 4. a) How does a cold pack work? Does it use an endothermic or an exothermic reaction? b Hot Pack Cold Pack Exothermic And Endothermic Processes Tell students that scientists describe temperature changes that occur when substances interact as either endothermic or exothermic. Tell students that they will explore how. In an endothermic reaction, a substance takes heat from its surroundings. The sodium thiosulfate needs energy to dissolve, so. Exothermic and endothermic reactions are all around us. Explore endothermic and exothermic process by making a cold. Hot Pack Cold Pack Exothermic And Endothermic Processes.
From slideplayer.com
Chemistry An Introduction ppt download Hot Pack Cold Pack Exothermic And Endothermic Processes Exothermic and endothermic reactions are all around us. Allow students to feel the temperature change in an activated cold pack and an activated hot pack. They are required to determine what chemical reaction generates heat and is reusable. Explore endothermic and exothermic process by making a cold pack or hot pack. Students design and build a reusable heat pack. The. Hot Pack Cold Pack Exothermic And Endothermic Processes.