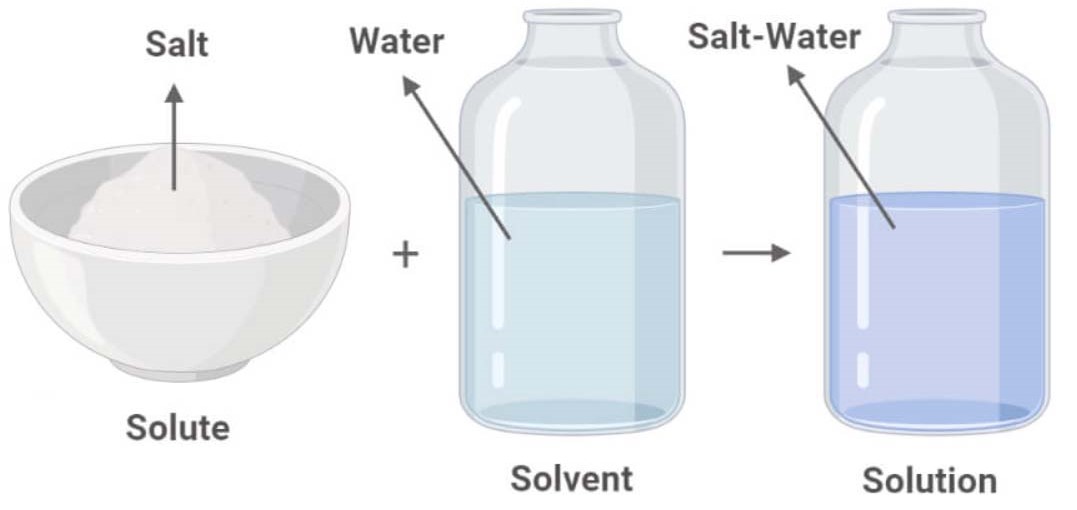
How Do Salt And Water Form A Solution . This is called a neutralization reaction and takes the following form: When a coloured solid dissolves, it. One example of a solution is salt water which is a mixture of water and salt. Sea water is a solution of salt dissolved into water., for example, salt or sugar in water. When acids and bases react with each other, they can form a salt and (usually) water. You cannot see the salt and the salt and water will stay a solution if left alone. A solubility curve shows how the solubility of a salt like sodium chloride or potassium nitrate varies with temperature. Solutions come in all phases, and the solvent and the solute do not have to be in the same phase to form a solution (such as salt and water).
from energyeducation.ca
Sea water is a solution of salt dissolved into water., for example, salt or sugar in water. Solutions come in all phases, and the solvent and the solute do not have to be in the same phase to form a solution (such as salt and water). One example of a solution is salt water which is a mixture of water and salt. This is called a neutralization reaction and takes the following form: When a coloured solid dissolves, it. When acids and bases react with each other, they can form a salt and (usually) water. You cannot see the salt and the salt and water will stay a solution if left alone. A solubility curve shows how the solubility of a salt like sodium chloride or potassium nitrate varies with temperature.
Solute Energy Education
How Do Salt And Water Form A Solution Solutions come in all phases, and the solvent and the solute do not have to be in the same phase to form a solution (such as salt and water). A solubility curve shows how the solubility of a salt like sodium chloride or potassium nitrate varies with temperature. When a coloured solid dissolves, it. One example of a solution is salt water which is a mixture of water and salt. Sea water is a solution of salt dissolved into water., for example, salt or sugar in water. Solutions come in all phases, and the solvent and the solute do not have to be in the same phase to form a solution (such as salt and water). When acids and bases react with each other, they can form a salt and (usually) water. You cannot see the salt and the salt and water will stay a solution if left alone. This is called a neutralization reaction and takes the following form:
From sciencenotes.org
How to Separate Sand and Salt How Do Salt And Water Form A Solution One example of a solution is salt water which is a mixture of water and salt. This is called a neutralization reaction and takes the following form: Sea water is a solution of salt dissolved into water., for example, salt or sugar in water. You cannot see the salt and the salt and water will stay a solution if left. How Do Salt And Water Form A Solution.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free How Do Salt And Water Form A Solution This is called a neutralization reaction and takes the following form: One example of a solution is salt water which is a mixture of water and salt. Sea water is a solution of salt dissolved into water., for example, salt or sugar in water. You cannot see the salt and the salt and water will stay a solution if left. How Do Salt And Water Form A Solution.
From www.slideserve.com
PPT Solute and Solvent PowerPoint Presentation ID433520 How Do Salt And Water Form A Solution When acids and bases react with each other, they can form a salt and (usually) water. One example of a solution is salt water which is a mixture of water and salt. You cannot see the salt and the salt and water will stay a solution if left alone. This is called a neutralization reaction and takes the following form:. How Do Salt And Water Form A Solution.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The How Do Salt And Water Form A Solution A solubility curve shows how the solubility of a salt like sodium chloride or potassium nitrate varies with temperature. When a coloured solid dissolves, it. This is called a neutralization reaction and takes the following form: Solutions come in all phases, and the solvent and the solute do not have to be in the same phase to form a solution. How Do Salt And Water Form A Solution.
From www.teachoo.com
Solution Definition, Types, Properties Chemistry Teachoo How Do Salt And Water Form A Solution One example of a solution is salt water which is a mixture of water and salt. Sea water is a solution of salt dissolved into water., for example, salt or sugar in water. A solubility curve shows how the solubility of a salt like sodium chloride or potassium nitrate varies with temperature. Solutions come in all phases, and the solvent. How Do Salt And Water Form A Solution.
From studiousguy.com
8 Coulomb’s Law Examples in Daily Life StudiousGuy How Do Salt And Water Form A Solution When acids and bases react with each other, they can form a salt and (usually) water. A solubility curve shows how the solubility of a salt like sodium chloride or potassium nitrate varies with temperature. You cannot see the salt and the salt and water will stay a solution if left alone. When a coloured solid dissolves, it. Sea water. How Do Salt And Water Form A Solution.
From byjus.com
You are provided with a mixture of sand, oil, salt, and water. Write How Do Salt And Water Form A Solution When a coloured solid dissolves, it. This is called a neutralization reaction and takes the following form: Solutions come in all phases, and the solvent and the solute do not have to be in the same phase to form a solution (such as salt and water). You cannot see the salt and the salt and water will stay a solution. How Do Salt And Water Form A Solution.
From energyeducation.ca
Solute Energy Education How Do Salt And Water Form A Solution This is called a neutralization reaction and takes the following form: A solubility curve shows how the solubility of a salt like sodium chloride or potassium nitrate varies with temperature. Solutions come in all phases, and the solvent and the solute do not have to be in the same phase to form a solution (such as salt and water). You. How Do Salt And Water Form A Solution.
From wou.edu
CH104 Chapter 7 Solutions Chemistry How Do Salt And Water Form A Solution You cannot see the salt and the salt and water will stay a solution if left alone. One example of a solution is salt water which is a mixture of water and salt. This is called a neutralization reaction and takes the following form: Sea water is a solution of salt dissolved into water., for example, salt or sugar in. How Do Salt And Water Form A Solution.
From techiescientist.com
Is Saltwater a Homogeneous or a Heterogeneous Mixture? Techiescientist How Do Salt And Water Form A Solution Sea water is a solution of salt dissolved into water., for example, salt or sugar in water. This is called a neutralization reaction and takes the following form: Solutions come in all phases, and the solvent and the solute do not have to be in the same phase to form a solution (such as salt and water). A solubility curve. How Do Salt And Water Form A Solution.
From welltech.com
Drinking Salt Water Benefits & Do You Need To? Welltech How Do Salt And Water Form A Solution Sea water is a solution of salt dissolved into water., for example, salt or sugar in water. When a coloured solid dissolves, it. One example of a solution is salt water which is a mixture of water and salt. When acids and bases react with each other, they can form a salt and (usually) water. Solutions come in all phases,. How Do Salt And Water Form A Solution.
From slideplayer.com
With your neighbor… Explain why saltwater is a mixture and NOT a How Do Salt And Water Form A Solution A solubility curve shows how the solubility of a salt like sodium chloride or potassium nitrate varies with temperature. This is called a neutralization reaction and takes the following form: When acids and bases react with each other, they can form a salt and (usually) water. One example of a solution is salt water which is a mixture of water. How Do Salt And Water Form A Solution.
From chem.libretexts.org
8.3 Precipitation Reactions Chemistry LibreTexts How Do Salt And Water Form A Solution Sea water is a solution of salt dissolved into water., for example, salt or sugar in water. This is called a neutralization reaction and takes the following form: One example of a solution is salt water which is a mixture of water and salt. You cannot see the salt and the salt and water will stay a solution if left. How Do Salt And Water Form A Solution.
From www.shutterstock.com
69,708 Sand salt and water 图片、库存照片和矢量图 Shutterstock How Do Salt And Water Form A Solution Sea water is a solution of salt dissolved into water., for example, salt or sugar in water. A solubility curve shows how the solubility of a salt like sodium chloride or potassium nitrate varies with temperature. When a coloured solid dissolves, it. This is called a neutralization reaction and takes the following form: You cannot see the salt and the. How Do Salt And Water Form A Solution.
From visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry How Do Salt And Water Form A Solution This is called a neutralization reaction and takes the following form: Sea water is a solution of salt dissolved into water., for example, salt or sugar in water. You cannot see the salt and the salt and water will stay a solution if left alone. A solubility curve shows how the solubility of a salt like sodium chloride or potassium. How Do Salt And Water Form A Solution.
From byjus.com
You are provided with a mixture of sand, oil, salt, and water. Write How Do Salt And Water Form A Solution When a coloured solid dissolves, it. When acids and bases react with each other, they can form a salt and (usually) water. You cannot see the salt and the salt and water will stay a solution if left alone. A solubility curve shows how the solubility of a salt like sodium chloride or potassium nitrate varies with temperature. One example. How Do Salt And Water Form A Solution.
From www.thoughtco.com
How to Separate Salt and Sand — 3 Methods How Do Salt And Water Form A Solution One example of a solution is salt water which is a mixture of water and salt. This is called a neutralization reaction and takes the following form: You cannot see the salt and the salt and water will stay a solution if left alone. A solubility curve shows how the solubility of a salt like sodium chloride or potassium nitrate. How Do Salt And Water Form A Solution.
From www.youtube.com
Dissolving Salt in Water YouTube How Do Salt And Water Form A Solution This is called a neutralization reaction and takes the following form: Solutions come in all phases, and the solvent and the solute do not have to be in the same phase to form a solution (such as salt and water). When a coloured solid dissolves, it. A solubility curve shows how the solubility of a salt like sodium chloride or. How Do Salt And Water Form A Solution.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID How Do Salt And Water Form A Solution You cannot see the salt and the salt and water will stay a solution if left alone. Sea water is a solution of salt dissolved into water., for example, salt or sugar in water. One example of a solution is salt water which is a mixture of water and salt. A solubility curve shows how the solubility of a salt. How Do Salt And Water Form A Solution.
From slideshare.net
Solution suspension How Do Salt And Water Form A Solution One example of a solution is salt water which is a mixture of water and salt. This is called a neutralization reaction and takes the following form: Solutions come in all phases, and the solvent and the solute do not have to be in the same phase to form a solution (such as salt and water). When acids and bases. How Do Salt And Water Form A Solution.
From www.alamy.com
Salt water in beaker experiment illustration Stock Vector Image & Art How Do Salt And Water Form A Solution Sea water is a solution of salt dissolved into water., for example, salt or sugar in water. Solutions come in all phases, and the solvent and the solute do not have to be in the same phase to form a solution (such as salt and water). When a coloured solid dissolves, it. When acids and bases react with each other,. How Do Salt And Water Form A Solution.
From www.nagwa.com
Question Video Identifying the Salt That Would Produce a Basic How Do Salt And Water Form A Solution A solubility curve shows how the solubility of a salt like sodium chloride or potassium nitrate varies with temperature. This is called a neutralization reaction and takes the following form: You cannot see the salt and the salt and water will stay a solution if left alone. One example of a solution is salt water which is a mixture of. How Do Salt And Water Form A Solution.
From pixels.com
Salt, Water And Solution Photograph by GIPhotoStock Images How Do Salt And Water Form A Solution You cannot see the salt and the salt and water will stay a solution if left alone. One example of a solution is salt water which is a mixture of water and salt. Solutions come in all phases, and the solvent and the solute do not have to be in the same phase to form a solution (such as salt. How Do Salt And Water Form A Solution.
From www.alamy.com
Solutions. homogeneous mixture. experiment with salt and water How Do Salt And Water Form A Solution When acids and bases react with each other, they can form a salt and (usually) water. One example of a solution is salt water which is a mixture of water and salt. Sea water is a solution of salt dissolved into water., for example, salt or sugar in water. This is called a neutralization reaction and takes the following form:. How Do Salt And Water Form A Solution.
From general.chemistrysteps.com
Acidity of a Salt Solution Chemistry Steps How Do Salt And Water Form A Solution A solubility curve shows how the solubility of a salt like sodium chloride or potassium nitrate varies with temperature. Solutions come in all phases, and the solvent and the solute do not have to be in the same phase to form a solution (such as salt and water). One example of a solution is salt water which is a mixture. How Do Salt And Water Form A Solution.
From brainly.in
The image shows particles of salt dissolved in water. Brainly.in How Do Salt And Water Form A Solution You cannot see the salt and the salt and water will stay a solution if left alone. This is called a neutralization reaction and takes the following form: When a coloured solid dissolves, it. When acids and bases react with each other, they can form a salt and (usually) water. Sea water is a solution of salt dissolved into water.,. How Do Salt And Water Form A Solution.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The How Do Salt And Water Form A Solution One example of a solution is salt water which is a mixture of water and salt. You cannot see the salt and the salt and water will stay a solution if left alone. When acids and bases react with each other, they can form a salt and (usually) water. A solubility curve shows how the solubility of a salt like. How Do Salt And Water Form A Solution.
From www.pinterest.com
Is Dissolving Salt in Water a Chemical Change or a Physical Change? in How Do Salt And Water Form A Solution Sea water is a solution of salt dissolved into water., for example, salt or sugar in water. One example of a solution is salt water which is a mixture of water and salt. This is called a neutralization reaction and takes the following form: When acids and bases react with each other, they can form a salt and (usually) water.. How Do Salt And Water Form A Solution.
From byjus.com
Suggest separation techniques one would need to employ to separate the How Do Salt And Water Form A Solution You cannot see the salt and the salt and water will stay a solution if left alone. Solutions come in all phases, and the solvent and the solute do not have to be in the same phase to form a solution (such as salt and water). When acids and bases react with each other, they can form a salt and. How Do Salt And Water Form A Solution.
From www.vrogue.co
Solved When Sodium Chloride Dissolves In Water The Re vrogue.co How Do Salt And Water Form A Solution When a coloured solid dissolves, it. Sea water is a solution of salt dissolved into water., for example, salt or sugar in water. A solubility curve shows how the solubility of a salt like sodium chloride or potassium nitrate varies with temperature. Solutions come in all phases, and the solvent and the solute do not have to be in the. How Do Salt And Water Form A Solution.
From sciencenotes.org
What Is a Salt in Chemistry? Definition and Examples How Do Salt And Water Form A Solution One example of a solution is salt water which is a mixture of water and salt. You cannot see the salt and the salt and water will stay a solution if left alone. A solubility curve shows how the solubility of a salt like sodium chloride or potassium nitrate varies with temperature. When a coloured solid dissolves, it. Sea water. How Do Salt And Water Form A Solution.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt How Do Salt And Water Form A Solution A solubility curve shows how the solubility of a salt like sodium chloride or potassium nitrate varies with temperature. You cannot see the salt and the salt and water will stay a solution if left alone. Solutions come in all phases, and the solvent and the solute do not have to be in the same phase to form a solution. How Do Salt And Water Form A Solution.
From www.nagwa.com
Question Video Identifying a Series of Methods to Separate Sodium How Do Salt And Water Form A Solution One example of a solution is salt water which is a mixture of water and salt. You cannot see the salt and the salt and water will stay a solution if left alone. When a coloured solid dissolves, it. Solutions come in all phases, and the solvent and the solute do not have to be in the same phase to. How Do Salt And Water Form A Solution.
From sciencemsqblog8.blogspot.com
Science8 Semester 2,Chapter 4 Mixtures How Do Salt And Water Form A Solution This is called a neutralization reaction and takes the following form: When acids and bases react with each other, they can form a salt and (usually) water. Sea water is a solution of salt dissolved into water., for example, salt or sugar in water. Solutions come in all phases, and the solvent and the solute do not have to be. How Do Salt And Water Form A Solution.
From byjus.com
Perform an activity to find out how to dissolve a solid in a liquid? How Do Salt And Water Form A Solution This is called a neutralization reaction and takes the following form: A solubility curve shows how the solubility of a salt like sodium chloride or potassium nitrate varies with temperature. Solutions come in all phases, and the solvent and the solute do not have to be in the same phase to form a solution (such as salt and water). When. How Do Salt And Water Form A Solution.