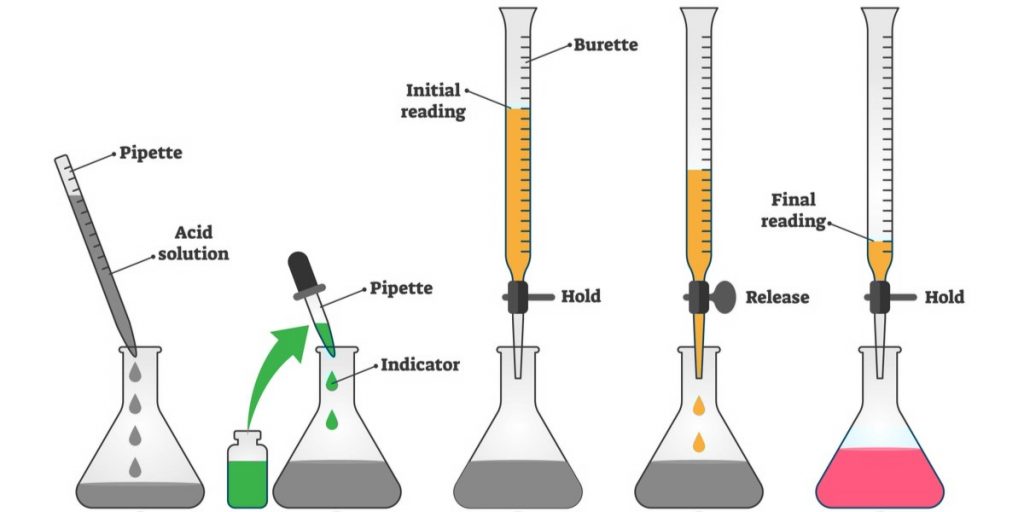
Why Base Is Taken In Burette . The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data. A titration is a very commonly used type of quantitative analysis. a buret is a glass apparatus for delivering precise, variable volumes of solution. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. It is primarily used for titration to. It is based on accurately. once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. when naoh (strong base) is added to hcl (strong acid), the equivalence point occurs at a ph of 7 (as seen in 1.3). Burettes are tall, thin, graduated glass tubes, with a tap at the bottom that can be opened and closed to allow the solution inside to flow out.
from www.microlit.us
when naoh (strong base) is added to hcl (strong acid), the equivalence point occurs at a ph of 7 (as seen in 1.3). a buret is a glass apparatus for delivering precise, variable volumes of solution. A titration is a very commonly used type of quantitative analysis. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. Burettes are tall, thin, graduated glass tubes, with a tap at the bottom that can be opened and closed to allow the solution inside to flow out. The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data. It is based on accurately. It is primarily used for titration to.
Uses of Burettes in Pharmaceutical Industry Diagrams & Functions
Why Base Is Taken In Burette when naoh (strong base) is added to hcl (strong acid), the equivalence point occurs at a ph of 7 (as seen in 1.3). The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data. It is primarily used for titration to. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. when naoh (strong base) is added to hcl (strong acid), the equivalence point occurs at a ph of 7 (as seen in 1.3). A titration is a very commonly used type of quantitative analysis. a buret is a glass apparatus for delivering precise, variable volumes of solution. once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. Burettes are tall, thin, graduated glass tubes, with a tap at the bottom that can be opened and closed to allow the solution inside to flow out. It is based on accurately.
From meaningkosh.com
How Does A Burette Work MeaningKosh Why Base Is Taken In Burette A titration is a very commonly used type of quantitative analysis. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. The \(co_2\). Why Base Is Taken In Burette.
From dxoiavyze.blob.core.windows.net
Base Burette Laboratory Apparatus Uses at Tiffany Miller blog Why Base Is Taken In Burette a buret is a glass apparatus for delivering precise, variable volumes of solution. once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution. Why Base Is Taken In Burette.
From www.youtube.com
Titration Step 2 Preparing the burette YouTube Why Base Is Taken In Burette when naoh (strong base) is added to hcl (strong acid), the equivalence point occurs at a ph of 7 (as seen in 1.3). It is primarily used for titration to. The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data. It is based on accurately. once you’ve. Why Base Is Taken In Burette.
From www.microlit.us
Explore What is a Burette, Its Uses, Functions, and Diagrams Why Base Is Taken In Burette It is based on accurately. once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. It is primarily used for titration to. Burettes are tall, thin, graduated glass tubes, with a tap at the bottom that can be opened and closed to allow the. Why Base Is Taken In Burette.
From www.nagwa.com
Question Video Explaining Why a Buret Should Not Be Clamped Too Why Base Is Taken In Burette It is based on accurately. A titration is a very commonly used type of quantitative analysis. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data. It is. Why Base Is Taken In Burette.
From www.alamy.com
Reading a burette. When reading a burette the reading is taken from the Why Base Is Taken In Burette when naoh (strong base) is added to hcl (strong acid), the equivalence point occurs at a ph of 7 (as seen in 1.3). a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. a buret is a glass apparatus for delivering precise, variable volumes of solution. It. Why Base Is Taken In Burette.
From dxojngeez.blob.core.windows.net
Burette And Accuracy at Jana Thompson blog Why Base Is Taken In Burette A titration is a very commonly used type of quantitative analysis. a buret is a glass apparatus for delivering precise, variable volumes of solution. The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data. It is primarily used for titration to. It is based on accurately. when. Why Base Is Taken In Burette.
From www.slideserve.com
PPT Volumetric Analysis AcidBase PowerPoint Presentation, free Why Base Is Taken In Burette It is primarily used for titration to. once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data. It is based on accurately. . Why Base Is Taken In Burette.
From sbl1023.blogspot.com
LAB 3 TITRATION Why Base Is Taken In Burette once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. Burettes are tall, thin, graduated glass tubes, with a tap at the bottom that can be opened and closed to allow the solution inside to flow out. The \(co_2\) forms carbonic acid (\(h_2co_3\)) when. Why Base Is Taken In Burette.
From dxoiavyze.blob.core.windows.net
Base Burette Laboratory Apparatus Uses at Tiffany Miller blog Why Base Is Taken In Burette once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. Burettes are tall, thin, graduated glass tubes, with a tap at the bottom that can be opened and closed to allow the solution inside to flow out. when naoh (strong base) is added. Why Base Is Taken In Burette.
From www.youtube.com
How to read a burette YouTube Why Base Is Taken In Burette once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data. Burettes are tall, thin, graduated glass tubes, with a tap at the bottom. Why Base Is Taken In Burette.
From exoynmahb.blob.core.windows.net
Alkali Base Burette at Johnny Su blog Why Base Is Taken In Burette a buret is a glass apparatus for delivering precise, variable volumes of solution. once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution. Why Base Is Taken In Burette.
From www.slideserve.com
PPT titrations PowerPoint Presentation, free download ID3456377 Why Base Is Taken In Burette It is primarily used for titration to. Burettes are tall, thin, graduated glass tubes, with a tap at the bottom that can be opened and closed to allow the solution inside to flow out. once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration.. Why Base Is Taken In Burette.
From dxoazcfwv.blob.core.windows.net
Buret Vs Burette at Veda Morris blog Why Base Is Taken In Burette It is primarily used for titration to. once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. Burettes are tall, thin, graduated glass tubes, with a tap at the bottom that can be opened and closed to allow the solution inside to flow out.. Why Base Is Taken In Burette.
From www.nagwa.com
Question Video Understanding the Risks Associated with Filling a Buret Why Base Is Taken In Burette when naoh (strong base) is added to hcl (strong acid), the equivalence point occurs at a ph of 7 (as seen in 1.3). a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. Burettes are tall, thin, graduated glass tubes, with a tap at the bottom that can. Why Base Is Taken In Burette.
From dxokrrpqe.blob.core.windows.net
What Is Burette And Pipette at Julio Ortega blog Why Base Is Taken In Burette when naoh (strong base) is added to hcl (strong acid), the equivalence point occurs at a ph of 7 (as seen in 1.3). a buret is a glass apparatus for delivering precise, variable volumes of solution. once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your. Why Base Is Taken In Burette.
From dxoiavyze.blob.core.windows.net
Base Burette Laboratory Apparatus Uses at Tiffany Miller blog Why Base Is Taken In Burette once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. when naoh (strong base) is added to hcl (strong acid), the equivalence point occurs at a ph of 7 (as seen in 1.3). It is primarily used for titration to. The \(co_2\) forms. Why Base Is Taken In Burette.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Why Base Is Taken In Burette Burettes are tall, thin, graduated glass tubes, with a tap at the bottom that can be opened and closed to allow the solution inside to flow out. The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data. a buret is a glass apparatus for delivering precise, variable volumes. Why Base Is Taken In Burette.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps Why Base Is Taken In Burette It is primarily used for titration to. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. A titration is a very commonly used type of quantitative analysis. The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data.. Why Base Is Taken In Burette.
From www.microlit.us
Explore What is a Burette, Its Uses, Functions, and Diagrams Why Base Is Taken In Burette A titration is a very commonly used type of quantitative analysis. a buret is a glass apparatus for delivering precise, variable volumes of solution. once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. Burettes are tall, thin, graduated glass tubes, with a. Why Base Is Taken In Burette.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Why Base Is Taken In Burette a buret is a glass apparatus for delivering precise, variable volumes of solution. The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data. It is primarily used for titration to. a buret is primarily used for titration to determine the concentration of an unknown solution by adding. Why Base Is Taken In Burette.
From dxojngeez.blob.core.windows.net
Burette And Accuracy at Jana Thompson blog Why Base Is Taken In Burette once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. A titration is a very commonly used type of quantitative analysis. when. Why Base Is Taken In Burette.
From yaletools.com
Burette Functions Types and How to Use Them YaleTools Why Base Is Taken In Burette It is primarily used for titration to. A titration is a very commonly used type of quantitative analysis. once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. when naoh (strong base) is added to hcl (strong acid), the equivalence point occurs at. Why Base Is Taken In Burette.
From mmerevise.co.uk
Acids and Bases Questions and Revision MME Why Base Is Taken In Burette It is primarily used for titration to. The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data. a buret is a glass apparatus for delivering precise, variable volumes of solution. a buret is primarily used for titration to determine the concentration of an unknown solution by adding. Why Base Is Taken In Burette.
From www.youtube.com
How to read a burette YouTube Why Base Is Taken In Burette once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. It is based on accurately. The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data. a buret is a glass apparatus for. Why Base Is Taken In Burette.
From www.studocu.com
Experiment 5 Introduction to the Buret and a Simple AcidBase Why Base Is Taken In Burette once you’ve got your acid of unknown concentration in the conical flask, it’s time to set up the burette with your alkali of known concentration. The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data. a buret is a glass apparatus for delivering precise, variable volumes of. Why Base Is Taken In Burette.
From www.microlit.us
Uses of Burettes in Pharmaceutical Industry Diagrams & Functions Why Base Is Taken In Burette It is primarily used for titration to. It is based on accurately. The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. a buret is a glass. Why Base Is Taken In Burette.
From dxoazcfwv.blob.core.windows.net
Buret Vs Burette at Veda Morris blog Why Base Is Taken In Burette The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data. when naoh (strong base) is added to hcl (strong acid), the equivalence point occurs at a ph of 7 (as seen in 1.3). once you’ve got your acid of unknown concentration in the conical flask, it’s time. Why Base Is Taken In Burette.
From www.slideserve.com
PPT WarmUp PowerPoint Presentation, free download ID2515685 Why Base Is Taken In Burette It is primarily used for titration to. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. A titration is a very commonly used type of quantitative analysis. It is based on accurately. The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer,. Why Base Is Taken In Burette.
From chem.libretexts.org
Proper Use of a Buret Chemistry LibreTexts Why Base Is Taken In Burette when naoh (strong base) is added to hcl (strong acid), the equivalence point occurs at a ph of 7 (as seen in 1.3). Burettes are tall, thin, graduated glass tubes, with a tap at the bottom that can be opened and closed to allow the solution inside to flow out. once you’ve got your acid of unknown concentration. Why Base Is Taken In Burette.
From www.alamy.com
Burette for precision measurements in the chemistry laboratory Stock Why Base Is Taken In Burette a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. It is primarily used for titration to. a buret is a glass apparatus for delivering precise, variable volumes of solution. Burettes are tall, thin, graduated glass tubes, with a tap at the bottom that can be opened and. Why Base Is Taken In Burette.
From www.slideserve.com
PPT Buret PowerPoint Presentation, free download ID3095341 Why Base Is Taken In Burette It is based on accurately. when naoh (strong base) is added to hcl (strong acid), the equivalence point occurs at a ph of 7 (as seen in 1.3). a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. It is primarily used for titration to. a buret. Why Base Is Taken In Burette.
From poe.com
What is the typical solution placed in the burette during acidbase Why Base Is Taken In Burette when naoh (strong base) is added to hcl (strong acid), the equivalence point occurs at a ph of 7 (as seen in 1.3). Burettes are tall, thin, graduated glass tubes, with a tap at the bottom that can be opened and closed to allow the solution inside to flow out. It is primarily used for titration to. a. Why Base Is Taken In Burette.
From www.slideserve.com
PPT AcidBase Titration & pH Chapter 15 PowerPoint Presentation ID Why Base Is Taken In Burette a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. Burettes are tall, thin, graduated glass tubes, with a tap at the bottom that can be opened and closed to allow the solution inside to flow out. once you’ve got your acid of unknown concentration in the conical. Why Base Is Taken In Burette.
From www.dreamstime.com
Burette Chemistry Stock Illustrations 102 Burette Chemistry Stock Why Base Is Taken In Burette It is based on accurately. a buret is a glass apparatus for delivering precise, variable volumes of solution. The \(co_2\) forms carbonic acid (\(h_2co_3\)) when dissolved in water that then acts as a buffer, reducing the accuracy of data. A titration is a very commonly used type of quantitative analysis. when naoh (strong base) is added to hcl. Why Base Is Taken In Burette.