Crystals Definition Chemistry . State what is meant by a crystal's habit, and identify some factors that might affect it. Covalent crystals have covalent bonds. Because there are repeated units, crystals have recognizable structures. Crystals are solids in which all of the atoms are arranged in repeating patterns. Metallic crystals have metallic bonds. The word “crystal” comes from the greek word krustallos, which means both “rock. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Metallic, ionic, covalent, and molecular. Identify the three kinds of rotational symmetry axes of a cube. Ionic crystals have ionic bonds. However, liquid crystals also exist. Those solids in which atoms,. Crystals are also called crystalline solids because most crystals are solid. There are 4 types of crystals: Explain why the angles between adjacent faces (of even a broken crystal) tend to have the same small set of values.
from collegedunia.com
However, liquid crystals also exist. State what is meant by a crystal's habit, and identify some factors that might affect it. Covalent crystals have covalent bonds. There are 4 types of crystals: Because there are repeated units, crystals have recognizable structures. The lattice that forms extends out in three dimensions. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. The word “crystal” comes from the greek word krustallos, which means both “rock. Crystals are also called crystalline solids because most crystals are solid. Ionic crystals have ionic bonds.
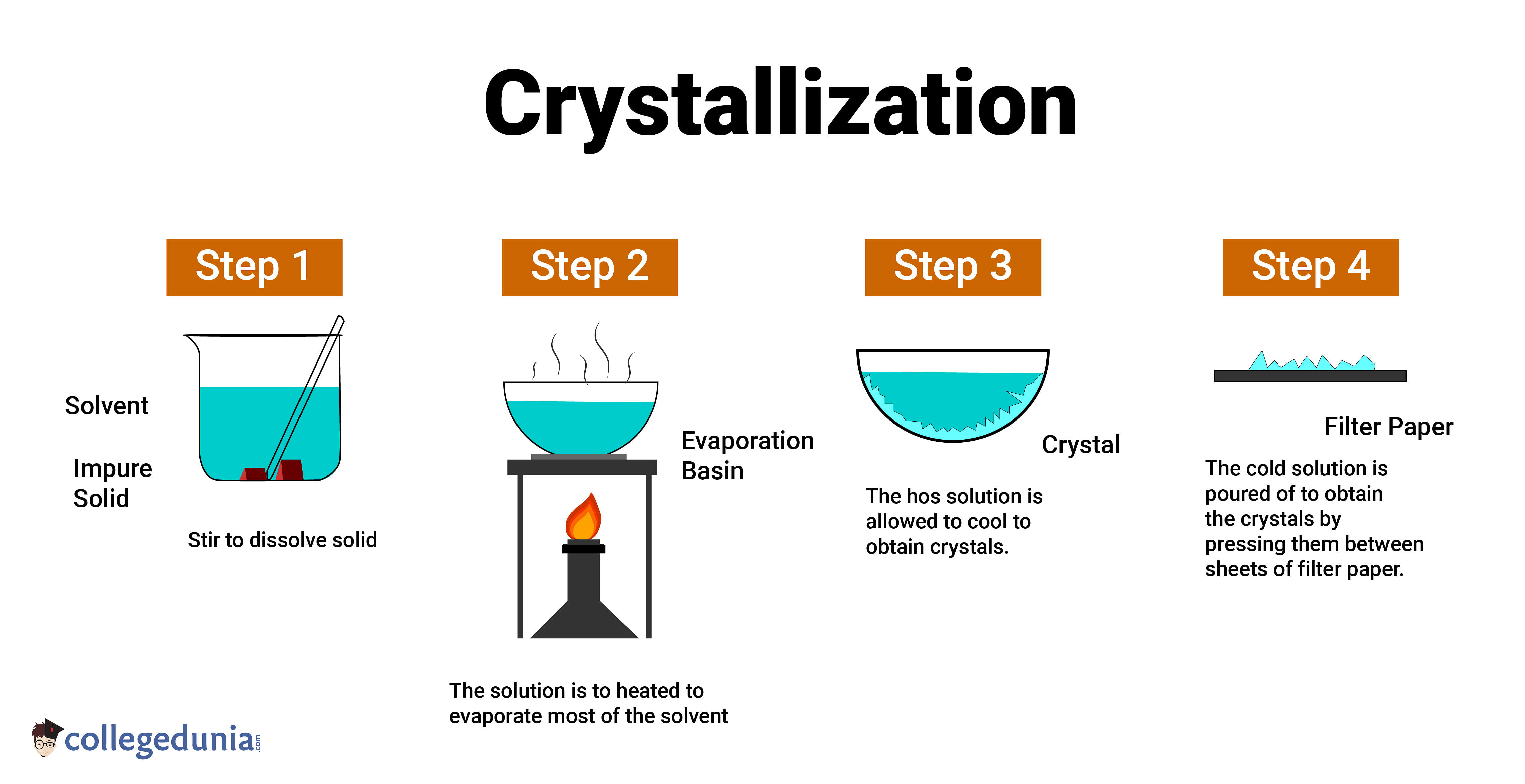
Crystallization Definition, Process, Types & Examples
Crystals Definition Chemistry Crystals are also called crystalline solids because most crystals are solid. Crystals are solids in which all of the atoms are arranged in repeating patterns. There are 4 types of crystals: State what is meant by a crystal's habit, and identify some factors that might affect it. Because there are repeated units, crystals have recognizable structures. Metallic crystals have metallic bonds. However, liquid crystals also exist. The lattice that forms extends out in three dimensions. Identify the three kinds of rotational symmetry axes of a cube. Ionic crystals have ionic bonds. Explain why the angles between adjacent faces (of even a broken crystal) tend to have the same small set of values. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Crystals are also called crystalline solids because most crystals are solid. Metallic, ionic, covalent, and molecular. The word “crystal” comes from the greek word krustallos, which means both “rock. Covalent crystals have covalent bonds.
From collegedunia.com
Crystallization Definition, Process, Types & Examples Crystals Definition Chemistry Identify the three kinds of rotational symmetry axes of a cube. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Crystals are also called crystalline solids because most crystals are solid. Ionic crystals have ionic bonds. However, liquid crystals also exist. Covalent crystals have covalent bonds. Crystals are solids in which all. Crystals Definition Chemistry.
From www.findlight.net
XRay Diffraction Getting to Know Crystal Structures (Part Ⅰ) Crystals Definition Chemistry Explain why the angles between adjacent faces (of even a broken crystal) tend to have the same small set of values. State what is meant by a crystal's habit, and identify some factors that might affect it. There are 4 types of crystals: However, liquid crystals also exist. A crystal consists of matter that is formed from an ordered arrangement. Crystals Definition Chemistry.
From www.slideserve.com
PPT Introduction to Crystallization Chemistry PowerPoint Presentation Crystals Definition Chemistry Those solids in which atoms,. However, liquid crystals also exist. The word “crystal” comes from the greek word krustallos, which means both “rock. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Covalent crystals have covalent bonds. State what is meant by a crystal's habit, and identify some factors that might affect. Crystals Definition Chemistry.
From www.slideserve.com
PPT Chapter 1 Crystal Structure PowerPoint Presentation, free Crystals Definition Chemistry However, liquid crystals also exist. Ionic crystals have ionic bonds. Covalent crystals have covalent bonds. Because there are repeated units, crystals have recognizable structures. Explain why the angles between adjacent faces (of even a broken crystal) tend to have the same small set of values. Metallic, ionic, covalent, and molecular. The lattice that forms extends out in three dimensions. There. Crystals Definition Chemistry.
From chemistnotes.com
Covalent crystals, Definition, Example, and Properties Chemistry Notes Crystals Definition Chemistry State what is meant by a crystal's habit, and identify some factors that might affect it. The word “crystal” comes from the greek word krustallos, which means both “rock. However, liquid crystals also exist. Metallic crystals have metallic bonds. Explain why the angles between adjacent faces (of even a broken crystal) tend to have the same small set of values.. Crystals Definition Chemistry.
From www.slideserve.com
PPT Fundamentals of crystal chemistry PowerPoint Presentation, free Crystals Definition Chemistry Because there are repeated units, crystals have recognizable structures. The lattice that forms extends out in three dimensions. Explain why the angles between adjacent faces (of even a broken crystal) tend to have the same small set of values. Metallic, ionic, covalent, and molecular. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or. Crystals Definition Chemistry.
From chem.libretexts.org
Crystalline Solid Structures Chemistry LibreTexts Crystals Definition Chemistry The lattice that forms extends out in three dimensions. Crystals are solids in which all of the atoms are arranged in repeating patterns. The word “crystal” comes from the greek word krustallos, which means both “rock. Crystals are also called crystalline solids because most crystals are solid. Because there are repeated units, crystals have recognizable structures. However, liquid crystals also. Crystals Definition Chemistry.
From study.com
How Atoms & Molecules Form Solids Patterns & Crystals Video & Lesson Crystals Definition Chemistry There are 4 types of crystals: Crystals are solids in which all of the atoms are arranged in repeating patterns. State what is meant by a crystal's habit, and identify some factors that might affect it. Because there are repeated units, crystals have recognizable structures. Covalent crystals have covalent bonds. Those solids in which atoms,. Metallic crystals have metallic bonds.. Crystals Definition Chemistry.
From chemistnotes.com
Covalent crystals, Definition, Example, and Properties Chemistry Notes Crystals Definition Chemistry The word “crystal” comes from the greek word krustallos, which means both “rock. There are 4 types of crystals: Metallic crystals have metallic bonds. State what is meant by a crystal's habit, and identify some factors that might affect it. Covalent crystals have covalent bonds. The lattice that forms extends out in three dimensions. Crystals are solids in which all. Crystals Definition Chemistry.
From eduinput.com
Crystal lattice Definition, Types, examples, lattice point Crystals Definition Chemistry Crystals are solids in which all of the atoms are arranged in repeating patterns. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. The word “crystal” comes from the greek word krustallos, which means both “rock. Metallic crystals have metallic bonds. There are 4 types of crystals: However, liquid crystals also exist.. Crystals Definition Chemistry.
From www.slideserve.com
PPT Lecture 3 Rocks and Minerals PowerPoint Presentation, free Crystals Definition Chemistry The lattice that forms extends out in three dimensions. Ionic crystals have ionic bonds. Identify the three kinds of rotational symmetry axes of a cube. Covalent crystals have covalent bonds. The word “crystal” comes from the greek word krustallos, which means both “rock. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions.. Crystals Definition Chemistry.
From www.slideserve.com
PPT Definition of the crystalline state Crystals are solids (but not Crystals Definition Chemistry Crystals are also called crystalline solids because most crystals are solid. Metallic, ionic, covalent, and molecular. The lattice that forms extends out in three dimensions. Explain why the angles between adjacent faces (of even a broken crystal) tend to have the same small set of values. State what is meant by a crystal's habit, and identify some factors that might. Crystals Definition Chemistry.
From eduinput.com
Crystalline solids Properties, types, examples use Crystals Definition Chemistry The word “crystal” comes from the greek word krustallos, which means both “rock. Metallic, ionic, covalent, and molecular. Covalent crystals have covalent bonds. State what is meant by a crystal's habit, and identify some factors that might affect it. Crystals are also called crystalline solids because most crystals are solid. There are 4 types of crystals: Ionic crystals have ionic. Crystals Definition Chemistry.
From www.geologyin.com
Crystal Structure and Crystal Systems Crystals Definition Chemistry Crystals are also called crystalline solids because most crystals are solid. Metallic, ionic, covalent, and molecular. However, liquid crystals also exist. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. State what is meant by a crystal's habit, and identify some factors that might affect it. Those solids in which atoms,. Explain. Crystals Definition Chemistry.
From www.thoughtco.com
Crystal Definition, Examples, and Common Types Crystals Definition Chemistry State what is meant by a crystal's habit, and identify some factors that might affect it. Because there are repeated units, crystals have recognizable structures. Identify the three kinds of rotational symmetry axes of a cube. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Metallic, ionic, covalent, and molecular. Those solids. Crystals Definition Chemistry.
From www.thoughtco.com
What Is a Crystal in Chemistry? Crystals Definition Chemistry State what is meant by a crystal's habit, and identify some factors that might affect it. Identify the three kinds of rotational symmetry axes of a cube. Metallic crystals have metallic bonds. Those solids in which atoms,. The lattice that forms extends out in three dimensions. Because there are repeated units, crystals have recognizable structures. A crystal consists of matter. Crystals Definition Chemistry.
From www.ck12.org
Comparing Crystals Overview ( Video ) Chemistry CK12 Foundation Crystals Definition Chemistry There are 4 types of crystals: Crystals are also called crystalline solids because most crystals are solid. Metallic crystals have metallic bonds. Those solids in which atoms,. Explain why the angles between adjacent faces (of even a broken crystal) tend to have the same small set of values. The lattice that forms extends out in three dimensions. Because there are. Crystals Definition Chemistry.
From samson-blogjenkins.blogspot.com
Measurements Used to Describe Crystal Structures Crystals Definition Chemistry A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Crystals are also called crystalline solids because most crystals are solid. However, liquid crystals also exist. State what is meant by a crystal's habit, and identify some factors that might affect it. Covalent crystals have covalent bonds. Identify the three kinds of rotational. Crystals Definition Chemistry.
From www.slideserve.com
PPT Crystallography Basics PowerPoint Presentation, free download Crystals Definition Chemistry The lattice that forms extends out in three dimensions. Ionic crystals have ionic bonds. Explain why the angles between adjacent faces (of even a broken crystal) tend to have the same small set of values. Crystals are also called crystalline solids because most crystals are solid. Metallic, ionic, covalent, and molecular. Covalent crystals have covalent bonds. A crystal consists of. Crystals Definition Chemistry.
From www.slideserve.com
PPT Chapter 1 Crystal Structure PowerPoint Presentation, free Crystals Definition Chemistry There are 4 types of crystals: Identify the three kinds of rotational symmetry axes of a cube. Metallic, ionic, covalent, and molecular. Explain why the angles between adjacent faces (of even a broken crystal) tend to have the same small set of values. State what is meant by a crystal's habit, and identify some factors that might affect it. Covalent. Crystals Definition Chemistry.
From chem.libretexts.org
12.6 Crystal Structures Chemistry LibreTexts Crystals Definition Chemistry Ionic crystals have ionic bonds. Metallic, ionic, covalent, and molecular. Metallic crystals have metallic bonds. Crystals are solids in which all of the atoms are arranged in repeating patterns. However, liquid crystals also exist. The word “crystal” comes from the greek word krustallos, which means both “rock. State what is meant by a crystal's habit, and identify some factors that. Crystals Definition Chemistry.
From opengeology.org
4 Crystals and Crystallization Mineralogy Crystals Definition Chemistry Explain why the angles between adjacent faces (of even a broken crystal) tend to have the same small set of values. Those solids in which atoms,. Identify the three kinds of rotational symmetry axes of a cube. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. However, liquid crystals also exist. The. Crystals Definition Chemistry.
From www.youtube.com
What are different types of crystals. Solid State Physical Crystals Definition Chemistry Explain why the angles between adjacent faces (of even a broken crystal) tend to have the same small set of values. Ionic crystals have ionic bonds. State what is meant by a crystal's habit, and identify some factors that might affect it. There are 4 types of crystals: However, liquid crystals also exist. Metallic, ionic, covalent, and molecular. Those solids. Crystals Definition Chemistry.
From www.britannica.com
crystal Types of bonds Britannica Crystals Definition Chemistry Metallic crystals have metallic bonds. The word “crystal” comes from the greek word krustallos, which means both “rock. Ionic crystals have ionic bonds. Identify the three kinds of rotational symmetry axes of a cube. Covalent crystals have covalent bonds. State what is meant by a crystal's habit, and identify some factors that might affect it. However, liquid crystals also exist.. Crystals Definition Chemistry.
From mungfali.com
Crystal Formation Types Crystals Definition Chemistry The word “crystal” comes from the greek word krustallos, which means both “rock. Because there are repeated units, crystals have recognizable structures. Those solids in which atoms,. Metallic, ionic, covalent, and molecular. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Covalent crystals have covalent bonds. There are 4 types of crystals:. Crystals Definition Chemistry.
From www.youtube.com
Unit 1.3 Definition of Crystals and Anisotropy YouTube Crystals Definition Chemistry Because there are repeated units, crystals have recognizable structures. The lattice that forms extends out in three dimensions. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Crystals are solids in which all of the atoms are arranged in repeating patterns. Metallic crystals have metallic bonds. State what is meant by a. Crystals Definition Chemistry.
From www.slideserve.com
PPT Mineral/Crystal Chemistry and Classification of Minerals Crystals Definition Chemistry However, liquid crystals also exist. Metallic crystals have metallic bonds. Crystals are solids in which all of the atoms are arranged in repeating patterns. The lattice that forms extends out in three dimensions. The word “crystal” comes from the greek word krustallos, which means both “rock. Identify the three kinds of rotational symmetry axes of a cube. Because there are. Crystals Definition Chemistry.
From www.beautifulchemistry.net
Crystal Structure — Beautiful Chemistry Crystals Definition Chemistry Crystals are solids in which all of the atoms are arranged in repeating patterns. However, liquid crystals also exist. Identify the three kinds of rotational symmetry axes of a cube. Covalent crystals have covalent bonds. State what is meant by a crystal's habit, and identify some factors that might affect it. There are 4 types of crystals: The word “crystal”. Crystals Definition Chemistry.
From www.youtube.com
Lecture 1 Crystal Chemistry Introduction and crystal systems YouTube Crystals Definition Chemistry Ionic crystals have ionic bonds. Crystals are solids in which all of the atoms are arranged in repeating patterns. Crystals are also called crystalline solids because most crystals are solid. Because there are repeated units, crystals have recognizable structures. Those solids in which atoms,. The word “crystal” comes from the greek word krustallos, which means both “rock. However, liquid crystals. Crystals Definition Chemistry.
From opengeology.org
13 Crystal Structures Mineralogy Crystals Definition Chemistry Because there are repeated units, crystals have recognizable structures. There are 4 types of crystals: Crystals are also called crystalline solids because most crystals are solid. State what is meant by a crystal's habit, and identify some factors that might affect it. Metallic crystals have metallic bonds. The word “crystal” comes from the greek word krustallos, which means both “rock.. Crystals Definition Chemistry.
From courses.lumenlearning.com
Types of Crystals Boundless Chemistry Crystals Definition Chemistry Metallic crystals have metallic bonds. Metallic, ionic, covalent, and molecular. Ionic crystals have ionic bonds. Because there are repeated units, crystals have recognizable structures. Crystals are also called crystalline solids because most crystals are solid. The word “crystal” comes from the greek word krustallos, which means both “rock. However, liquid crystals also exist. State what is meant by a crystal's. Crystals Definition Chemistry.
From bernieralice.hyperphp.com
Types Of Crystal Lattice Structure bernieralice Crystals Definition Chemistry The word “crystal” comes from the greek word krustallos, which means both “rock. Explain why the angles between adjacent faces (of even a broken crystal) tend to have the same small set of values. Covalent crystals have covalent bonds. Metallic crystals have metallic bonds. Metallic, ionic, covalent, and molecular. Ionic crystals have ionic bonds. Because there are repeated units, crystals. Crystals Definition Chemistry.
From learn.careers360.com
Show the structure of crystalline, polycrystalline and amorphous Crystals Definition Chemistry Crystals are also called crystalline solids because most crystals are solid. The word “crystal” comes from the greek word krustallos, which means both “rock. Explain why the angles between adjacent faces (of even a broken crystal) tend to have the same small set of values. There are 4 types of crystals: The lattice that forms extends out in three dimensions.. Crystals Definition Chemistry.
From www.thoughtco.com
Types of Crystals Shapes and Structures Crystals Definition Chemistry Those solids in which atoms,. State what is meant by a crystal's habit, and identify some factors that might affect it. The lattice that forms extends out in three dimensions. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Covalent crystals have covalent bonds. Explain why the angles between adjacent faces (of. Crystals Definition Chemistry.
From www.instructables.com
Chemistry Crystals! 4 Steps (with Pictures) Instructables Crystals Definition Chemistry The word “crystal” comes from the greek word krustallos, which means both “rock. A crystal consists of matter that is formed from an ordered arrangement of atoms, molecules, or ions. Explain why the angles between adjacent faces (of even a broken crystal) tend to have the same small set of values. Crystals are solids in which all of the atoms. Crystals Definition Chemistry.