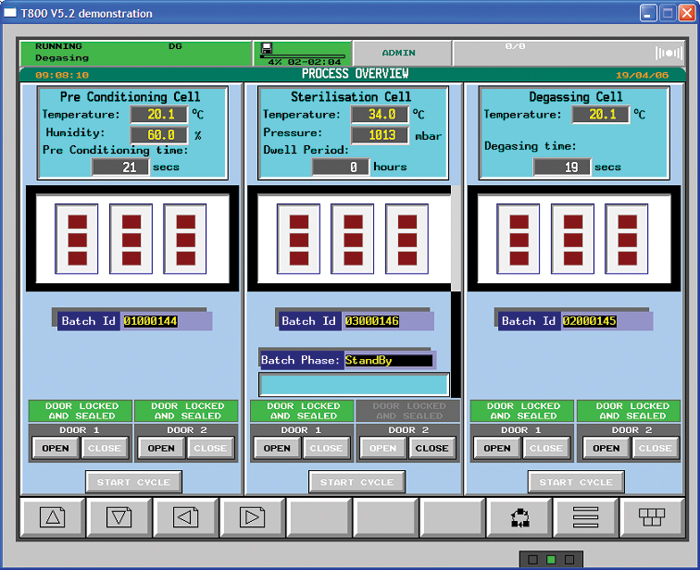
Eto Sterilization Guidelines . Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. And, highlight the importance and interrelationship of other. Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. The four essential parameters (operational ranges) are: Gas concentration (450 to 1200 mg/l); Learn more about eo sterilization here. This guide offers a deep dive into eto sterilization,. Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical.
from www.eurotherm.com
Learn more about eo sterilization here. And, highlight the importance and interrelationship of other. This guide offers a deep dive into eto sterilization,. Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. The four essential parameters (operational ranges) are: Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; Gas concentration (450 to 1200 mg/l); Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993.
Ethylene Oxide (EtO) Sterilization Process Eurotherm Limited
Eto Sterilization Guidelines Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Learn more about eo sterilization here. Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. And, highlight the importance and interrelationship of other. Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; The four essential parameters (operational ranges) are: For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. Gas concentration (450 to 1200 mg/l); This guide offers a deep dive into eto sterilization,.
From www.youtube.com
All About ETO Sterilization With ETO Sterilizer YouTube Eto Sterilization Guidelines Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. Learn more about eo sterilization here. For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. The four essential parameters (operational ranges) are: Gas concentration (450 to 1200 mg/l); Iso 11135:2014 specifies requirements for the development,. Eto Sterilization Guidelines.
From www.hospitalsterilizers.com
ETO Sterilization Monitoring in the Operation Theatre Ensuring Quality Eto Sterilization Guidelines The four essential parameters (operational ranges) are: Gas concentration (450 to 1200 mg/l); Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; And, highlight the importance and interrelationship of other. Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. For ethylene oxide sterilization, two voluntary consensus standards. Eto Sterilization Guidelines.
From www.hospitalsterilizers.com
Role of ETO Sterilizers in Preventing Infections in Hyderabad's Eto Sterilization Guidelines And, highlight the importance and interrelationship of other. Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. Gas concentration (450 to 1200 mg/l); Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; This guide offers a deep dive into eto sterilization,. Learn more about eo sterilization here.. Eto Sterilization Guidelines.
From www.steris.in
EtO Sterilization An Effective Method for Sterilizing Complex Medical Eto Sterilization Guidelines Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Learn more about eo sterilization here. This guide offers a deep dive into eto sterilization,. The four essential parameters (operational ranges) are: Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; Despite its efficacy, recent concerns. Eto Sterilization Guidelines.
From www.slideshare.net
ETO Sterilizer And Sterilization process Eto Sterilization Guidelines And, highlight the importance and interrelationship of other. For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; This guide offers a deep dive into eto sterilization,. Iso 11135:2014 specifies requirements for the development, validation and routine control of. Eto Sterilization Guidelines.
From www.centerpiece.life
The Crucial Role of ETO Sterilization in Medical Device Manufacturing Eto Sterilization Guidelines Gas concentration (450 to 1200 mg/l); This guide offers a deep dive into eto sterilization,. Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. And, highlight the importance and interrelationship of other. Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; For ethylene oxide sterilization, two voluntary. Eto Sterilization Guidelines.
From www.healthtard.com
Sterilization in Hospitals Eto Sterilization Guidelines For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. The four essential parameters (operational ranges) are: This guide offers a deep dive into eto sterilization,. Gas concentration (450 to 1200 mg/l); Learn more about eo sterilization here. Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; And,. Eto Sterilization Guidelines.
From www.slideserve.com
PPT Disinfection and Sterilization Guidelines What You Need to Know Eto Sterilization Guidelines And, highlight the importance and interrelationship of other. For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. Learn more about eo sterilization here. This guide offers a deep dive into eto sterilization,. Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Gas. Eto Sterilization Guidelines.
From www.slideserve.com
PPT eto sterilizer, eto sterilization, industrial eto sterilizer, eto Eto Sterilization Guidelines For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. And, highlight the importance and interrelationship of other. Gas concentration (450 to 1200 mg/l); This guide offers a deep dive into eto sterilization,. Iso 11135:2014 specifies. Eto Sterilization Guidelines.
From www.rsd-engineering.com
Protocols and validation EtO sterilization equipment RSD sterilization Eto Sterilization Guidelines Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; Gas concentration (450 to 1200 mg/l); The four essential parameters (operational ranges) are: Learn more about eo sterilization here. And, highlight the importance and interrelationship of other. Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. This guide. Eto Sterilization Guidelines.
From www.eurotherm.com
Ethylene Oxide (EtO) Sterilization Process Eurotherm Limited Eto Sterilization Guidelines The four essential parameters (operational ranges) are: Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. And, highlight the. Eto Sterilization Guidelines.
From www.hospitalsterilizers.com
ETO Sterilizer Role for Modern Needs Eto Sterilization Guidelines Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. This guide offers a deep dive into eto sterilization,. Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Gas. Eto Sterilization Guidelines.
From nimhansnews.online
The Complete Guide to ETO Sterilization in 2023 Benefits, Process, and Eto Sterilization Guidelines Learn more about eo sterilization here. Gas concentration (450 to 1200 mg/l); The four essential parameters (operational ranges) are: Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Guide development and implementation of. Eto Sterilization Guidelines.
From pt.slideshare.net
ETO Sterilizer And Sterilization process Eto Sterilization Guidelines Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; Learn more about eo sterilization here. This guide offers a deep dive into eto sterilization,. Gas concentration (450 to 1200 mg/l); The four essential parameters (operational ranges) are: For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. Despite. Eto Sterilization Guidelines.
From mavink.com
Ethylene Oxide Sterilization Process Eto Sterilization Guidelines Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. The four essential parameters (operational ranges) are: This guide offers a deep dive into eto sterilization,. And, highlight the importance and interrelationship of other.. Eto Sterilization Guidelines.
From mavink.com
Eto Sterilization Process Flow Chart Eto Sterilization Guidelines Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Learn more about eo sterilization here. The four essential parameters (operational ranges) are: Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi. Eto Sterilization Guidelines.
From www.scribd.com
ETO Sterilization Validation PDF Sterilization (Microbiology Eto Sterilization Guidelines Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. This guide offers a deep dive into eto sterilization,. Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. And,. Eto Sterilization Guidelines.
From www.slideserve.com
PPT Introduction to eto sterilizer and Sterilization process Eto Sterilization Guidelines Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. Learn more about eo sterilization here. Gas concentration (450 to 1200 mg/l); Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; And, highlight the importance and interrelationship of other. For ethylene oxide sterilization, two voluntary consensus standards (ansi. Eto Sterilization Guidelines.
From www.slideserve.com
PPT Introduction to eto sterilizer and Sterilization process Eto Sterilization Guidelines The four essential parameters (operational ranges) are: This guide offers a deep dive into eto sterilization,. And, highlight the importance and interrelationship of other. Gas concentration (450 to 1200 mg/l); Learn more about eo sterilization here. Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. For ethylene oxide sterilization, two. Eto Sterilization Guidelines.
From www.scribd.com
EMEA EtO Guideline PDF Sterilization (Microbiology) Chemistry Eto Sterilization Guidelines For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; Gas concentration (450 to 1200 mg/l); The four essential parameters (operational ranges). Eto Sterilization Guidelines.
From sterigenics.com
Ethylene Oxide Sterilization ETO Sterilization Services Eto Sterilization Guidelines Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. And, highlight the importance and interrelationship of other. For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. The four essential parameters (operational ranges) are: Learn more about eo sterilization here. Gas concentration (450 to 1200. Eto Sterilization Guidelines.
From www.scribd.com
Introduction to EtO Sterilization Sterilization (Microbiology Eto Sterilization Guidelines The four essential parameters (operational ranges) are: For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. Learn more about eo sterilization here. Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; Gas concentration (450 to 1200 mg/l); And, highlight the importance and interrelationship of other. Despite its. Eto Sterilization Guidelines.
From www.slideshare.net
Eto Sterilizer Ethylene Oxide (EtO) Sterilization Process Eto Sterilization Guidelines Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; And, highlight the importance and interrelationship of other. The four essential parameters (operational ranges) are: This guide offers a deep dive into eto sterilization,. Learn more about eo sterilization. Eto Sterilization Guidelines.
From ruilabels.com
ETO Sterilization Label Chemical Indicator Labels Ruilabels Eto Sterilization Guidelines The four essential parameters (operational ranges) are: Learn more about eo sterilization here. For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. This guide offers a deep dive into eto sterilization,. Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Despite its. Eto Sterilization Guidelines.
From www.slideshare.net
410 05e checklist_sterilization_eto_iso111351 Eto Sterilization Guidelines Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Gas concentration (450 to 1200 mg/l); This guide offers a deep dive into eto sterilization,. Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. For ethylene oxide sterilization, two voluntary consensus standards (ansi. Eto Sterilization Guidelines.
From www.slideserve.com
PPT Importance of ETO Sterilizer in Food Industry PowerPoint Eto Sterilization Guidelines Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. This guide offers a deep dive into eto sterilization,. And, highlight the importance and interrelationship of other. Learn more about eo sterilization here. The four essential parameters (operational ranges). Eto Sterilization Guidelines.
From www.adinath.co.in
ETO Sterilization Process for Industrial Applications Eto Sterilization Guidelines And, highlight the importance and interrelationship of other. Learn more about eo sterilization here. For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; This guide offers a deep dive into eto sterilization,. Iso 11135:2014 specifies requirements for the. Eto Sterilization Guidelines.
From tsquality.ch
Understanding the Ethylene Oxide (EtO) Sterilization TSQuality.ch Eto Sterilization Guidelines Gas concentration (450 to 1200 mg/l); This guide offers a deep dive into eto sterilization,. And, highlight the importance and interrelationship of other. The four essential parameters (operational ranges) are: Learn more about eo sterilization here. Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. Guide development and implementation of parametric release. Eto Sterilization Guidelines.
From mavink.com
Eto Sterilization Process Flow Chart Eto Sterilization Guidelines And, highlight the importance and interrelationship of other. The four essential parameters (operational ranges) are: Learn more about eo sterilization here. Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; Gas concentration (450 to 1200 mg/l); For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. This guide. Eto Sterilization Guidelines.
From www.rsd-engineering.com
Ethylene oxide sterilization Process RSD Industrial sterilization Eto Sterilization Guidelines Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. And, highlight the importance and interrelationship of other. For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. Gas concentration (450 to 1200 mg/l); This guide offers a deep dive into eto sterilization,. Despite. Eto Sterilization Guidelines.
From wfhss-guidelines.com
Sterilization Wfhss Guidelines Eto Sterilization Guidelines Learn more about eo sterilization here. For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. And, highlight the importance and interrelationship of other. Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; Gas concentration (450 to 1200 mg/l); The four essential parameters (operational ranges) are: This guide. Eto Sterilization Guidelines.
From www.slideserve.com
PPT Introduction to eto sterilizer and Sterilization process Eto Sterilization Guidelines Gas concentration (450 to 1200 mg/l); Learn more about eo sterilization here. Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; And, highlight the importance and interrelationship of other. Iso 11135:2014 specifies requirements for the development, validation and. Eto Sterilization Guidelines.
From www.regulatorymedicaldevice.com
ETO Sterilization Sterilization of Health Care Products Using Eto Sterilization Guidelines For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. Gas concentration (450 to 1200 mg/l); Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Learn more about eo sterilization here. Guide development and implementation of parametric release for a new ethylene oxide. Eto Sterilization Guidelines.
From www.steris.in
STERIS India's First Outsourcing ETO Sterilization Service Eto Sterilization Guidelines For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; Despite its efficacy, recent concerns over environmental and user safety have cast a spotlight on eto sterilization. Learn more about eo sterilization here. The four essential parameters (operational ranges). Eto Sterilization Guidelines.
From bhsi.com.pk
ETO Commercial Sterilization B & H Surgical Instrument Eto Sterilization Guidelines This guide offers a deep dive into eto sterilization,. Guide development and implementation of parametric release for a new ethylene oxide sterilization cycle; The four essential parameters (operational ranges) are: For ethylene oxide sterilization, two voluntary consensus standards (ansi aami iso 11135:2014 and ansi aami iso 10993. Learn more about eo sterilization here. Iso 11135:2014 specifies requirements for the development,. Eto Sterilization Guidelines.