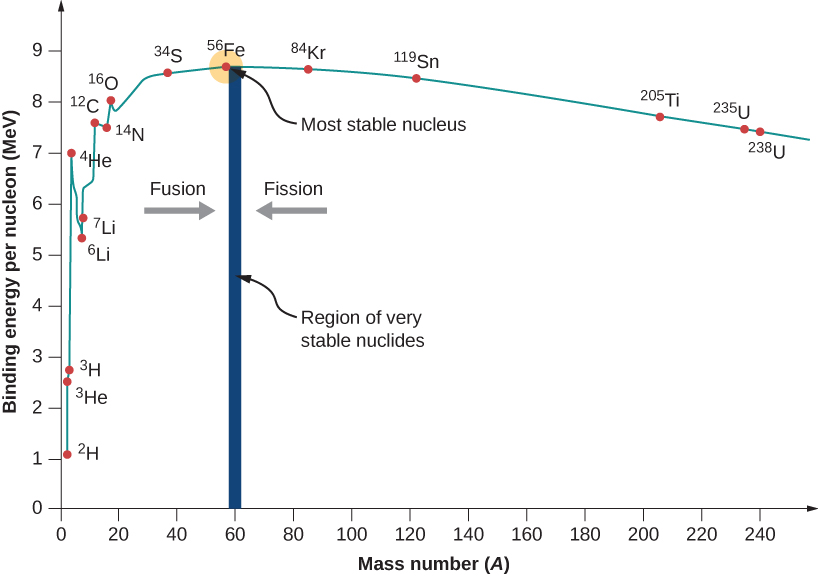
An Isotope With High Binding Energy Per Nucleon . Use the graph to identify each isotope’s binding energy per nucleon. Binding energy = binding energy per nucleon × mass number. In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. This quantity is the average energy. Elements with a high binding energy per. Calculate the binding energy per nucleon of a particle. The more tightly bound a system is, the stronger the forces that hold it together and the greater the energy required to pull it apart. The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by. The graph below (figure 1) shows the binding energy per nucleon against nucleon number. Determine the binding energy of each isotope. However if you plot binding.
from phys.libretexts.org
Binding energy = binding energy per nucleon × mass number. The graph below (figure 1) shows the binding energy per nucleon against nucleon number. Elements with a high binding energy per. Determine the binding energy of each isotope. Use the graph to identify each isotope’s binding energy per nucleon. This quantity is the average energy. In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by. The more tightly bound a system is, the stronger the forces that hold it together and the greater the energy required to pull it apart. Calculate the binding energy per nucleon of a particle.
12.3 Nuclear Binding Energy Physics LibreTexts
An Isotope With High Binding Energy Per Nucleon Use the graph to identify each isotope’s binding energy per nucleon. This quantity is the average energy. Binding energy = binding energy per nucleon × mass number. The more tightly bound a system is, the stronger the forces that hold it together and the greater the energy required to pull it apart. The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by. The graph below (figure 1) shows the binding energy per nucleon against nucleon number. However if you plot binding. Determine the binding energy of each isotope. Calculate the binding energy per nucleon of a particle. In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. Elements with a high binding energy per. Use the graph to identify each isotope’s binding energy per nucleon.
From www.researchgate.net
The binding energies per nucleon of Zr and Sn isotopes. The results An Isotope With High Binding Energy Per Nucleon In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. Use the graph to identify each isotope’s binding energy per nucleon. However if you plot binding. Calculate the binding energy per nucleon of a particle. This quantity is the average energy. Elements with a high binding energy per. Binding. An Isotope With High Binding Energy Per Nucleon.
From www.slideserve.com
PPT Nuclear Physics PowerPoint Presentation, free download ID1985809 An Isotope With High Binding Energy Per Nucleon The more tightly bound a system is, the stronger the forces that hold it together and the greater the energy required to pull it apart. This quantity is the average energy. Use the graph to identify each isotope’s binding energy per nucleon. Elements with a high binding energy per. In nuclear physics, one of the most important experimental quantities is. An Isotope With High Binding Energy Per Nucleon.
From www.researchgate.net
The binding energy per nucleon (BE/A) in MeV for isotopes of Z = 119 An Isotope With High Binding Energy Per Nucleon This quantity is the average energy. Determine the binding energy of each isotope. The more tightly bound a system is, the stronger the forces that hold it together and the greater the energy required to pull it apart. Calculate the binding energy per nucleon of a particle. However if you plot binding. Binding energy = binding energy per nucleon ×. An Isotope With High Binding Energy Per Nucleon.
From www.researchgate.net
1 Binding energy per nucleon for stable nuclei. The most tightly bound An Isotope With High Binding Energy Per Nucleon This quantity is the average energy. Binding energy = binding energy per nucleon × mass number. The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by. However if you plot binding. Elements with a high binding energy per. The graph below (figure 1) shows the binding energy. An Isotope With High Binding Energy Per Nucleon.
From www.researchgate.net
The binding energy per nucleon (BE/A) in MeV for isotopes of Z = 119 An Isotope With High Binding Energy Per Nucleon Determine the binding energy of each isotope. This quantity is the average energy. The more tightly bound a system is, the stronger the forces that hold it together and the greater the energy required to pull it apart. Use the graph to identify each isotope’s binding energy per nucleon. In nuclear physics, one of the most important experimental quantities is. An Isotope With High Binding Energy Per Nucleon.
From pages.swcp.com
Nucleotopes and the Nuclei of the Elemental Isotopes An Isotope With High Binding Energy Per Nucleon Binding energy = binding energy per nucleon × mass number. Elements with a high binding energy per. However if you plot binding. Calculate the binding energy per nucleon of a particle. This quantity is the average energy. The graph below (figure 1) shows the binding energy per nucleon against nucleon number. The more tightly bound a system is, the stronger. An Isotope With High Binding Energy Per Nucleon.
From www.slideserve.com
PPT Outline Chapter 8a The Nucleus PowerPoint Presentation ID671719 An Isotope With High Binding Energy Per Nucleon This quantity is the average energy. Binding energy = binding energy per nucleon × mass number. The graph below (figure 1) shows the binding energy per nucleon against nucleon number. Calculate the binding energy per nucleon of a particle. Elements with a high binding energy per. However if you plot binding. The relative stability of a nucleus is correlated with. An Isotope With High Binding Energy Per Nucleon.
From www.researchgate.net
Binding energies per nucleon (upper part) of Mg isotopes. The An Isotope With High Binding Energy Per Nucleon Determine the binding energy of each isotope. The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by. Calculate the binding energy per nucleon of a particle. This quantity is the average energy. Binding energy = binding energy per nucleon × mass number. Elements with a high binding. An Isotope With High Binding Energy Per Nucleon.
From www.slideserve.com
PPT Nuclear Binding Energy PowerPoint Presentation, free download An Isotope With High Binding Energy Per Nucleon In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. The more tightly bound a system is, the stronger the forces that hold it together and the greater the energy required to pull it apart. Calculate the binding energy per nucleon of a particle. Determine the binding energy of. An Isotope With High Binding Energy Per Nucleon.
From www.slideserve.com
PPT Chapter 31 Nuclear Physics PowerPoint Presentation, free An Isotope With High Binding Energy Per Nucleon Calculate the binding energy per nucleon of a particle. The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by. However if you plot binding. This quantity is the average energy. Use the graph to identify each isotope’s binding energy per nucleon. Binding energy = binding energy per. An Isotope With High Binding Energy Per Nucleon.
From www.slideserve.com
PPT Binding Energy PowerPoint Presentation, free download ID1150105 An Isotope With High Binding Energy Per Nucleon Elements with a high binding energy per. Calculate the binding energy per nucleon of a particle. However if you plot binding. The graph below (figure 1) shows the binding energy per nucleon against nucleon number. Use the graph to identify each isotope’s binding energy per nucleon. Binding energy = binding energy per nucleon × mass number. The relative stability of. An Isotope With High Binding Energy Per Nucleon.
From www.slideserve.com
PPT Chapter 21 Nuclear Chemistry PowerPoint Presentation, free An Isotope With High Binding Energy Per Nucleon Elements with a high binding energy per. Calculate the binding energy per nucleon of a particle. In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. This quantity is the average energy. The graph below (figure 1) shows the binding energy per nucleon against nucleon number. The relative stability. An Isotope With High Binding Energy Per Nucleon.
From www.britannica.com
Nuclear binding energy Definition, Formula, Mass Defect, & Graph An Isotope With High Binding Energy Per Nucleon In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. Determine the binding energy of each isotope. Calculate the binding energy per nucleon of a particle. However if you plot binding. This quantity is the average energy. The relative stability of a nucleus is correlated with its binding energy. An Isotope With High Binding Energy Per Nucleon.
From www.chegg.com
Solved calculate the binding energy per nucleon for a An Isotope With High Binding Energy Per Nucleon Use the graph to identify each isotope’s binding energy per nucleon. Binding energy = binding energy per nucleon × mass number. Elements with a high binding energy per. The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by. However if you plot binding. The graph below (figure. An Isotope With High Binding Energy Per Nucleon.
From www.researchgate.net
The binding energy per nucleon of neutron rich Niisotopes with mass An Isotope With High Binding Energy Per Nucleon Calculate the binding energy per nucleon of a particle. The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by. Determine the binding energy of each isotope. Elements with a high binding energy per. However if you plot binding. Binding energy = binding energy per nucleon × mass. An Isotope With High Binding Energy Per Nucleon.
From www.researchgate.net
3 Plot of the binding energy per nucleon for the most stable isotope of An Isotope With High Binding Energy Per Nucleon The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by. In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. The more tightly bound a system is, the stronger the forces that hold it together and. An Isotope With High Binding Energy Per Nucleon.
From www.researchgate.net
3 Plot of the binding energy per nucleon for the most stable isotope of An Isotope With High Binding Energy Per Nucleon This quantity is the average energy. Elements with a high binding energy per. Use the graph to identify each isotope’s binding energy per nucleon. The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by. The more tightly bound a system is, the stronger the forces that hold. An Isotope With High Binding Energy Per Nucleon.
From marketbusinessnews.com
The difference between nuclear fission and nuclear fusion An Isotope With High Binding Energy Per Nucleon The more tightly bound a system is, the stronger the forces that hold it together and the greater the energy required to pull it apart. The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by. Binding energy = binding energy per nucleon × mass number. The graph. An Isotope With High Binding Energy Per Nucleon.
From www.researchgate.net
Calculated binding energies per nucleon for Hg, Pb and Po isotopes An Isotope With High Binding Energy Per Nucleon In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. Calculate the binding energy per nucleon of a particle. The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by. Binding energy = binding energy per nucleon. An Isotope With High Binding Energy Per Nucleon.
From www.researchgate.net
Binding energy per nucleon versus atomic mass number showing the An Isotope With High Binding Energy Per Nucleon Use the graph to identify each isotope’s binding energy per nucleon. Calculate the binding energy per nucleon of a particle. Binding energy = binding energy per nucleon × mass number. Determine the binding energy of each isotope. However if you plot binding. This quantity is the average energy. The graph below (figure 1) shows the binding energy per nucleon against. An Isotope With High Binding Energy Per Nucleon.
From www.youtube.com
Binding Energy per Nucleon and Stability IB Physics YouTube An Isotope With High Binding Energy Per Nucleon Binding energy = binding energy per nucleon × mass number. Elements with a high binding energy per. The graph below (figure 1) shows the binding energy per nucleon against nucleon number. However if you plot binding. In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. Calculate the binding. An Isotope With High Binding Energy Per Nucleon.
From chemistry.stackexchange.com
isotope Which element has the highest binding energy per nucleon An Isotope With High Binding Energy Per Nucleon The more tightly bound a system is, the stronger the forces that hold it together and the greater the energy required to pull it apart. Calculate the binding energy per nucleon of a particle. Determine the binding energy of each isotope. Binding energy = binding energy per nucleon × mass number. The relative stability of a nucleus is correlated with. An Isotope With High Binding Energy Per Nucleon.
From www.slideserve.com
PPT Nuclear notation and Binding energy Contents Atomic notation An Isotope With High Binding Energy Per Nucleon This quantity is the average energy. However if you plot binding. Elements with a high binding energy per. Binding energy = binding energy per nucleon × mass number. The more tightly bound a system is, the stronger the forces that hold it together and the greater the energy required to pull it apart. The relative stability of a nucleus is. An Isotope With High Binding Energy Per Nucleon.
From www.researchgate.net
3 Plot of the binding energy per nucleon for the most stable isotope of An Isotope With High Binding Energy Per Nucleon Use the graph to identify each isotope’s binding energy per nucleon. The graph below (figure 1) shows the binding energy per nucleon against nucleon number. Elements with a high binding energy per. In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. Determine the binding energy of each isotope.. An Isotope With High Binding Energy Per Nucleon.
From freeinternetstudy.com
How does binding energy per nucleon affect the stability of a nucleus An Isotope With High Binding Energy Per Nucleon In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. However if you plot binding. The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by. Calculate the binding energy per nucleon of a particle. The more. An Isotope With High Binding Energy Per Nucleon.
From www.toppr.com
Draw a plot of the binding energy per nucleon as a function of mass An Isotope With High Binding Energy Per Nucleon The graph below (figure 1) shows the binding energy per nucleon against nucleon number. Calculate the binding energy per nucleon of a particle. Use the graph to identify each isotope’s binding energy per nucleon. The more tightly bound a system is, the stronger the forces that hold it together and the greater the energy required to pull it apart. The. An Isotope With High Binding Energy Per Nucleon.
From curiophysics.com
Binding Energy Per Nucleon Binding Energy Curve » Curio Physics An Isotope With High Binding Energy Per Nucleon The graph below (figure 1) shows the binding energy per nucleon against nucleon number. In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. Calculate the binding energy per nucleon of a particle. However if you plot binding. Use the graph to identify each isotope’s binding energy per nucleon.. An Isotope With High Binding Energy Per Nucleon.
From www.researchgate.net
Binding energies per nucleon of Si isotopes as a function of the An Isotope With High Binding Energy Per Nucleon The more tightly bound a system is, the stronger the forces that hold it together and the greater the energy required to pull it apart. Determine the binding energy of each isotope. The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by. Elements with a high binding. An Isotope With High Binding Energy Per Nucleon.
From phys.libretexts.org
12.3 Nuclear Binding Energy Physics LibreTexts An Isotope With High Binding Energy Per Nucleon Calculate the binding energy per nucleon of a particle. Use the graph to identify each isotope’s binding energy per nucleon. Elements with a high binding energy per. The graph below (figure 1) shows the binding energy per nucleon against nucleon number. This quantity is the average energy. The more tightly bound a system is, the stronger the forces that hold. An Isotope With High Binding Energy Per Nucleon.
From www.numerade.com
SOLVED Calculate the binding energy and the binding energy per An Isotope With High Binding Energy Per Nucleon The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by. This quantity is the average energy. Binding energy = binding energy per nucleon × mass number. The more tightly bound a system is, the stronger the forces that hold it together and the greater the energy required. An Isotope With High Binding Energy Per Nucleon.
From www.researchgate.net
(Color online) Binding energy per nucleon (a) and the total binding An Isotope With High Binding Energy Per Nucleon The graph below (figure 1) shows the binding energy per nucleon against nucleon number. The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by. Binding energy = binding energy per nucleon × mass number. Elements with a high binding energy per. In nuclear physics, one of the. An Isotope With High Binding Energy Per Nucleon.
From www.slideshare.net
binding energy per nucleon An Isotope With High Binding Energy Per Nucleon In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. This quantity is the average energy. The relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the nucleus divided by. Elements with a high binding energy per. Binding energy =. An Isotope With High Binding Energy Per Nucleon.
From www.toppr.com
Verify the binding energy per nucleon given in Table421 the plutonium An Isotope With High Binding Energy Per Nucleon This quantity is the average energy. However if you plot binding. The graph below (figure 1) shows the binding energy per nucleon against nucleon number. In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. Determine the binding energy of each isotope. Use the graph to identify each isotope’s. An Isotope With High Binding Energy Per Nucleon.
From www.researchgate.net
Binding energies per nucleon (upper part) of Mg isotopes. The An Isotope With High Binding Energy Per Nucleon In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. However if you plot binding. Determine the binding energy of each isotope. The more tightly bound a system is, the stronger the forces that hold it together and the greater the energy required to pull it apart. This quantity. An Isotope With High Binding Energy Per Nucleon.
From www.slideserve.com
PPT Fission and fusion PowerPoint Presentation, free download ID An Isotope With High Binding Energy Per Nucleon In nuclear physics, one of the most important experimental quantities is the binding energy per nucleon (ben), which is defined by. Calculate the binding energy per nucleon of a particle. Determine the binding energy of each isotope. Use the graph to identify each isotope’s binding energy per nucleon. The relative stability of a nucleus is correlated with its binding energy. An Isotope With High Binding Energy Per Nucleon.