Nitrogen Heat Of Combustion . standard heat of combustion : Chemical, physical and thermal properties of nitrogen: standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. the phase diagram of nitrogen is shown below the table. Values at 25 o c (77 o f, 298 k) and. The energy liberated when a substance x undergoes complete combustion, with excess of. the calorific value of a sample, also known as its heat of combustion, is defined as the amount of heat released.
from www.slideserve.com
standard heat of combustion : 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. The energy liberated when a substance x undergoes complete combustion, with excess of. the phase diagram of nitrogen is shown below the table. Values at 25 o c (77 o f, 298 k) and. Chemical, physical and thermal properties of nitrogen: the calorific value of a sample, also known as its heat of combustion, is defined as the amount of heat released. the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of.
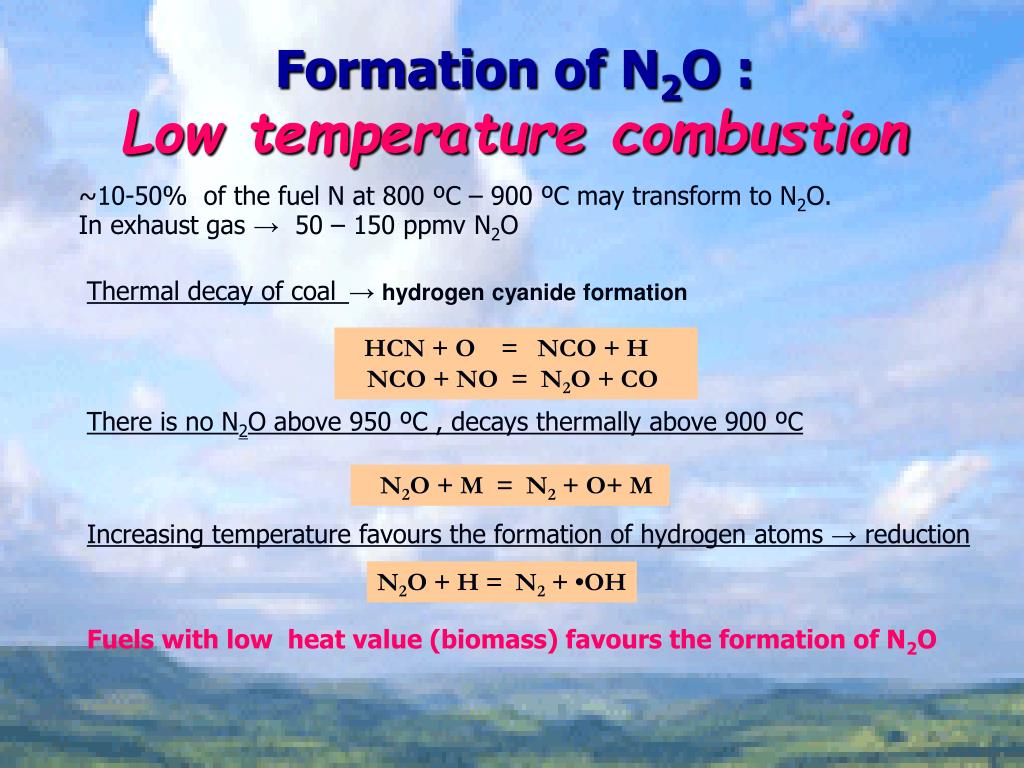
PPT NITROGENOXIDES PowerPoint Presentation, free download ID4735299
Nitrogen Heat Of Combustion The energy liberated when a substance x undergoes complete combustion, with excess of. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. The energy liberated when a substance x undergoes complete combustion, with excess of. the phase diagram of nitrogen is shown below the table. Chemical, physical and thermal properties of nitrogen: Values at 25 o c (77 o f, 298 k) and. the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. the calorific value of a sample, also known as its heat of combustion, is defined as the amount of heat released. standard heat of combustion :
From www.researchgate.net
Figure B.42 (a) Nitrogen density and (b) constant pressure specific Nitrogen Heat Of Combustion the phase diagram of nitrogen is shown below the table. Chemical, physical and thermal properties of nitrogen: the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. The energy liberated when a substance x undergoes complete combustion, with excess of. standard enthalpy of. Nitrogen Heat Of Combustion.
From socratic.org
How does nitrogen cycle through the biosphere? Socratic Nitrogen Heat Of Combustion the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. the calorific value of a sample, also known as its heat of combustion, is defined as the amount of heat released. Values at 25 o c (77 o f, 298 k) and. 84. Nitrogen Heat Of Combustion.
From www.processsensing.com
Nitrogen Production in Hazardous Areas Nitrogen Heat Of Combustion standard heat of combustion : 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. The energy liberated when a substance x undergoes complete combustion, with excess of. the phase diagram of nitrogen is shown below the table. the calorific value of a sample,. Nitrogen Heat Of Combustion.
From www.researchgate.net
A simplified scheme of nitrogen oxide formation during coal combustion Nitrogen Heat Of Combustion standard heat of combustion : the phase diagram of nitrogen is shown below the table. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. the calorific value of a sample, also known as its heat of combustion, is defined as the amount of heat released. the. Nitrogen Heat Of Combustion.
From www.aeronomie.be
Nitrogen dioxide NO2, a pollutant gas, released during combustion Nitrogen Heat Of Combustion standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. standard heat of combustion : Values at 25 o c (77 o f, 298 k) and. the phase diagram of nitrogen is shown below the table. the calorific value of a sample, also known as its heat of. Nitrogen Heat Of Combustion.
From www.researchgate.net
Output of nitrogen oxides in the combined combustion of gas and fuel Nitrogen Heat Of Combustion standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. Chemical, physical and thermal properties of nitrogen: standard heat of combustion : The energy liberated when a substance x undergoes complete combustion, with excess of. the phase diagram of nitrogen is shown below the table. 84 rows by. Nitrogen Heat Of Combustion.
From www.chegg.com
Solved 2. The boiling temperature of nitrogen at atmospheric Nitrogen Heat Of Combustion standard heat of combustion : the phase diagram of nitrogen is shown below the table. The energy liberated when a substance x undergoes complete combustion, with excess of. the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. Chemical, physical and thermal properties. Nitrogen Heat Of Combustion.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas Enthalpy Of Formation Nitrogen Heat Of Combustion The energy liberated when a substance x undergoes complete combustion, with excess of. the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. standard heat. Nitrogen Heat Of Combustion.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas Heat Of Formation Nitrogen Heat Of Combustion standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. Values at 25 o c (77 o f, 298 k) and. the calorific value of a sample, also known as its heat of combustion, is defined as the amount of heat released. 84 rows by convention, the (higher) heat. Nitrogen Heat Of Combustion.
From www.youtube.com
A2 Biology Reactions in the nitrogen cycle (OCR A Chapter 23.3) YouTube Nitrogen Heat Of Combustion the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. the calorific value of a sample, also known as its heat of combustion, is defined as the amount of heat released. 84 rows by convention, the (higher) heat of combustion is defined to. Nitrogen Heat Of Combustion.
From www.chegg.com
Solved Gaseous Nitrogen Dioxide NO2, Reacts With Oxygen T... Nitrogen Heat Of Combustion 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. standard heat of combustion : the calorific value of a sample, also known as its heat of combustion, is defined as the amount of heat released. The energy liberated when a substance x undergoes complete. Nitrogen Heat Of Combustion.
From www.slideserve.com
PPT NITROGENOXIDES PowerPoint Presentation, free download ID4735299 Nitrogen Heat Of Combustion 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. the phase diagram of nitrogen is shown below the table. the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. . Nitrogen Heat Of Combustion.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas Heat Of Formation Nitrogen Heat Of Combustion the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. the calorific value of a sample, also known as its heat. Nitrogen Heat Of Combustion.
From mackenzie-has-york.blogspot.com
Boiling Point of Nitrogen MackenziehasYork Nitrogen Heat Of Combustion 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. Values at 25 o c (77 o f, 298 k) and. the calorific value of a sample, also known as its heat of combustion, is defined as the amount of heat released. the heat of. Nitrogen Heat Of Combustion.
From www.researchgate.net
Oxygen, nitrogen and argon vapor pressure curves. Download Scientific Nitrogen Heat Of Combustion standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. the calorific value of a sample, also known as its heat of combustion, is defined as the. Nitrogen Heat Of Combustion.
From dokumen.tips
(PDF) NITROGEN OXIDES FORMATION in combustion processes DOKUMEN.TIPS Nitrogen Heat Of Combustion The energy liberated when a substance x undergoes complete combustion, with excess of. the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of. Nitrogen Heat Of Combustion.
From www.britannica.com
How the Nitrogen Cycle Works Britannica Nitrogen Heat Of Combustion The energy liberated when a substance x undergoes complete combustion, with excess of. Values at 25 o c (77 o f, 298 k) and. the calorific value of a sample, also known as its heat of combustion, is defined as the amount of heat released. the heat of combustion of a substance, also known as the calorific value. Nitrogen Heat Of Combustion.
From www.researchgate.net
Saturation curve for nitrogen. Download Scientific Diagram Nitrogen Heat Of Combustion the phase diagram of nitrogen is shown below the table. the calorific value of a sample, also known as its heat of combustion, is defined as the amount of heat released. standard heat of combustion : standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. 84. Nitrogen Heat Of Combustion.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Heat Capacity Of Nitrogen Gas Nitrogen Heat Of Combustion standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. standard heat of combustion : The energy liberated when a substance x undergoes complete combustion,. Nitrogen Heat Of Combustion.
From classes.oc.edu
THE LATENT HEAT OF VAPORIZATION OF NITROGEN Nitrogen Heat Of Combustion the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. the phase diagram of nitrogen is shown below the table. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. the calorific value of. Nitrogen Heat Of Combustion.
From exopjnllr.blob.core.windows.net
Two Types Of Combustion at Angela Pearce blog Nitrogen Heat Of Combustion the calorific value of a sample, also known as its heat of combustion, is defined as the amount of heat released. Chemical, physical and thermal properties of nitrogen: standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. the heat of combustion of a substance, also known as the. Nitrogen Heat Of Combustion.
From www.chemistrystudent.com
Alkanes (ALevel) ChemistryStudent Nitrogen Heat Of Combustion 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. the phase diagram of nitrogen is shown below the table. the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. Chemical,. Nitrogen Heat Of Combustion.
From www.researchgate.net
Experiment setup of near pseudocritical nitrogen heat transfer in Nitrogen Heat Of Combustion the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. The energy liberated when a substance x undergoes complete combustion, with excess of. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of. Nitrogen Heat Of Combustion.
From byjus.com
A gaseous mixture contains nitrogen and oxygen in the ratio of 4 1 by Nitrogen Heat Of Combustion the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. the phase diagram of nitrogen is shown below the table. The energy liberated when a. Nitrogen Heat Of Combustion.
From www.slideserve.com
PPT Combustion Reactions PowerPoint Presentation, free download ID Nitrogen Heat Of Combustion standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. Chemical, physical and thermal properties of nitrogen: the calorific value of a sample, also known as its heat of combustion, is defined as the amount of heat released. Values at 25 o c (77 o f, 298 k) and. . Nitrogen Heat Of Combustion.
From www.engineeringtoolbox.com
Nitrogen Enthalpy, Internal Energy and Entropy vs. Temperature Nitrogen Heat Of Combustion Chemical, physical and thermal properties of nitrogen: the phase diagram of nitrogen is shown below the table. the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. the calorific value of a sample, also known as its heat of combustion, is defined as. Nitrogen Heat Of Combustion.
From www.chegg.com
Solved Question 1 The boiling temperature of nitrogen at Nitrogen Heat Of Combustion the phase diagram of nitrogen is shown below the table. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. standard heat of combustion : The energy liberated when a substance x undergoes complete combustion, with excess of. standard enthalpy of combustio n (\(δh_c^\circ\)). Nitrogen Heat Of Combustion.
From www.scribd.com
Nitrogen Thermo JPCA 16 PDF Combustion Chemistry Nitrogen Heat Of Combustion the calorific value of a sample, also known as its heat of combustion, is defined as the amount of heat released. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. the phase diagram of nitrogen is shown below the table. 84 rows by convention, the (higher) heat. Nitrogen Heat Of Combustion.
From www.semanticscholar.org
Combustion Chemistry of Nitrogen Semantic Scholar Nitrogen Heat Of Combustion Values at 25 o c (77 o f, 298 k) and. the phase diagram of nitrogen is shown below the table. The energy liberated when a substance x undergoes complete combustion, with excess of. Chemical, physical and thermal properties of nitrogen: standard heat of combustion : 84 rows by convention, the (higher) heat of combustion is defined. Nitrogen Heat Of Combustion.
From marielatinrobertson.blogspot.com
Standard Enthalpy of Combustion MarielatinRobertson Nitrogen Heat Of Combustion the phase diagram of nitrogen is shown below the table. the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. Values at 25 o c (77 o f, 298 k) and. Chemical, physical and thermal properties of nitrogen: The energy liberated when a substance. Nitrogen Heat Of Combustion.
From www.earth.com
Why is the Nitrogen Cycle So Important? Nitrogen Heat Of Combustion the phase diagram of nitrogen is shown below the table. standard heat of combustion : the calorific value of a sample, also known as its heat of combustion, is defined as the amount of heat released. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. 84. Nitrogen Heat Of Combustion.
From pubs.rsc.org
The heats of combustion of organic compounds of nitrogen. Part 1. Ethyl Nitrogen Heat Of Combustion The energy liberated when a substance x undergoes complete combustion, with excess of. standard heat of combustion : the calorific value of a sample, also known as its heat of combustion, is defined as the amount of heat released. standard enthalpy of combustio n (\(δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines.. Nitrogen Heat Of Combustion.
From www.earth.com
Why is the Nitrogen Cycle So Important? Nitrogen Heat Of Combustion the heat of combustion of a substance, also known as the calorific value or the energy value, can be defined as the amount of. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. The energy liberated when a substance x undergoes complete combustion, with excess. Nitrogen Heat Of Combustion.
From www.researchgate.net
Species leaf nitrogen, ash content, heat of combustion and mass per Nitrogen Heat Of Combustion the phase diagram of nitrogen is shown below the table. The energy liberated when a substance x undergoes complete combustion, with excess of. 84 rows by convention, the (higher) heat of combustion is defined to be the heat released for the complete combustion of a. the calorific value of a sample, also known as its heat of. Nitrogen Heat Of Combustion.
From studylib.net
HEAT OF VAPORIZATION OF NITROGEN ©By Lee Marek Nitrogen Heat Of Combustion the phase diagram of nitrogen is shown below the table. Values at 25 o c (77 o f, 298 k) and. the calorific value of a sample, also known as its heat of combustion, is defined as the amount of heat released. The energy liberated when a substance x undergoes complete combustion, with excess of. standard enthalpy. Nitrogen Heat Of Combustion.