Titration Basic Reaction . A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. The basic process involves adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. In an indicator based titration you add another chemical that changes. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. There are two basic types of acid base titrations, indicator and potentiometric.
from www.tutormyself.com
Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In an indicator based titration you add another chemical that changes. The basic process involves adding a. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. There are two basic types of acid base titrations, indicator and potentiometric. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base.
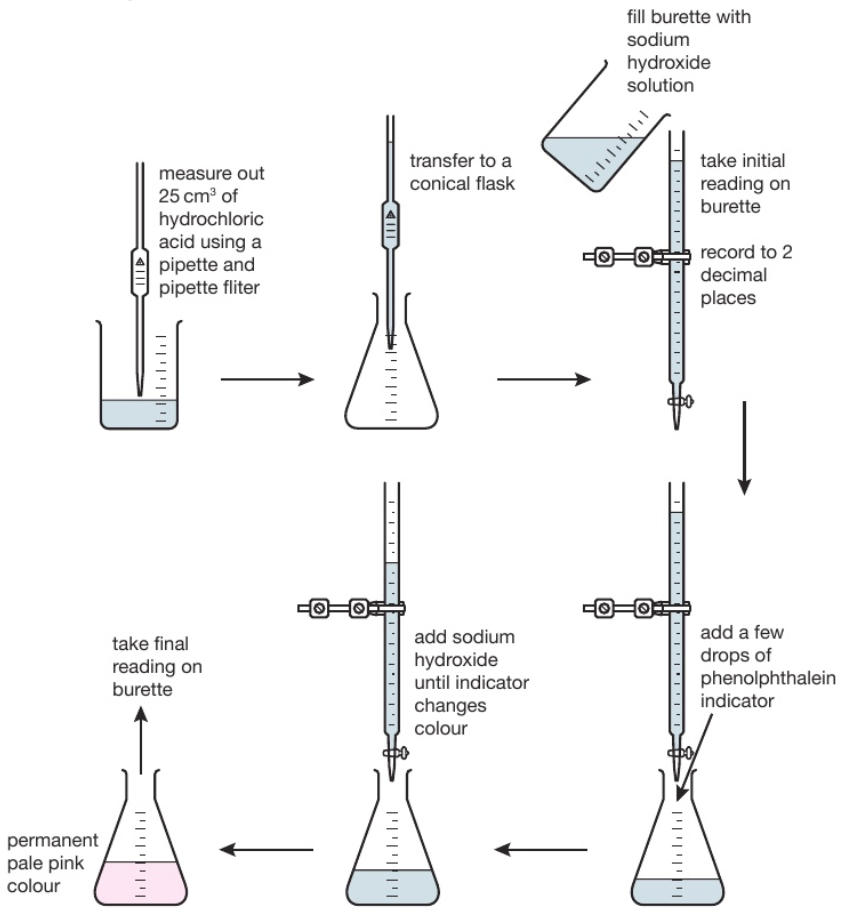
233 (Triple only) describe how to carry out an acidalkali titration
Titration Basic Reaction Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. In an indicator based titration you add another chemical that changes. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. The basic process involves adding a. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. There are two basic types of acid base titrations, indicator and potentiometric.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Basic Reaction In an indicator based titration you add another chemical that changes. The basic process involves adding a. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titration. Titration Basic Reaction.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Titration Basic Reaction Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. There are two basic types of acid base titrations, indicator and potentiometric. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined,. Titration Basic Reaction.
From mavink.com
Acid Base Titration Equation Titration Basic Reaction Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. There are two basic types of acid base titrations, indicator and potentiometric. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.. Titration Basic Reaction.
From www.science-revision.co.uk
Titrations Titration Basic Reaction Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. A chemical reaction is set up between a known volume of a solution. Titration Basic Reaction.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Titration Basic Reaction Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. There are two basic types of acid base titrations, indicator and potentiometric. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. A titration is a laboratory technique. Titration Basic Reaction.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Titration Basic Reaction The basic process involves adding a. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. Titration is a procedure used in chemistry. Titration Basic Reaction.
From www.youtube.com
WCLN Titrations Involving Precipitation Reactions Chemistry YouTube Titration Basic Reaction The basic process involves adding a. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A titration is a laboratory technique used to. Titration Basic Reaction.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Basic Reaction Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The basic process involves adding a. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A chemical reaction is set up between a known volume of. Titration Basic Reaction.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Basic Reaction There are two basic types of acid base titrations, indicator and potentiometric. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence. Titration Basic Reaction.
From bramblechemistry.weebly.com
4C6 Titration Titration Basic Reaction Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. In an indicator based titration you add another chemical that changes. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point. Titration Basic Reaction.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Basic Reaction Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. Titration is a procedure used. Titration Basic Reaction.
From www.youtube.com
REDOX REACTIONS AS THE BASIS FOR TITRATIONS YouTube Titration Basic Reaction In an indicator based titration you add another chemical that changes. There are two basic types of acid base titrations, indicator and potentiometric. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. Titration is a procedure used in chemistry in order. Titration Basic Reaction.
From tuitiontube.com
Details about AcidBase Titration Lab in Easy Language Tuition Tube Titration Basic Reaction Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. There are two basic types of acid base titrations, indicator and potentiometric. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. Titration. Titration Basic Reaction.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Basic Reaction Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known. Titration Basic Reaction.
From www.youtube.com
Basic Titrations Chemistry Tutorial YouTube Titration Basic Reaction Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. There are two basic types of acid base titrations, indicator and potentiometric. Titration is the slow addition of one solution of. Titration Basic Reaction.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration Basic Reaction There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes. The basic process involves adding a. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A chemical reaction is set up between a known volume of a. Titration Basic Reaction.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein Titration Basic Reaction A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. There are two basic types of acid base titrations, indicator. Titration Basic Reaction.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Basic Reaction A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. In an indicator based titration you add another chemical that changes. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has. Titration Basic Reaction.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Titration Basic Reaction In an indicator based titration you add another chemical that changes. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A chemical. Titration Basic Reaction.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Basic Reaction This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. The basic process involves adding a. In an indicator based titration you add another chemical that changes. Titration. Titration Basic Reaction.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 Titration Basic Reaction The basic process involves adding a. There are two basic types of acid base titrations, indicator and potentiometric. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In an indicator based titration you add another chemical that changes. This process continues until stoichiometrically equivalent amounts of the reactants have. Titration Basic Reaction.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID3080571 Titration Basic Reaction Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. In an indicator based titration you add another chemical that changes. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. Titration is the. Titration Basic Reaction.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Basic Reaction There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a. Titration Basic Reaction.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Basic Reaction Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. A chemical reaction is set up between a. Titration Basic Reaction.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Titration Basic Reaction Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. In an indicator based titration you add another chemical that changes. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point. Titration Basic Reaction.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Titration Basic Reaction The basic process involves adding a. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. There are two basic types of acid base titrations, indicator and potentiometric. Titration is a procedure used in chemistry in order to determine the molarity of an. Titration Basic Reaction.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Basic Reaction In an indicator based titration you add another chemical that changes. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. There are two basic types of acid base titrations, indicator and potentiometric. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known. Titration Basic Reaction.
From chem4three.blogspot.co.uk
CHEMISTRY 11 TITRATIONS Titration Basic Reaction The basic process involves adding a. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an. Titration Basic Reaction.
From chem.libretexts.org
17.3 AcidBase Titrations Chemistry LibreTexts Titration Basic Reaction In an indicator based titration you add another chemical that changes. There are two basic types of acid base titrations, indicator and potentiometric. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. Titration involves the gradual addition of a reagent of known concentration, known as the. Titration Basic Reaction.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Titration Basic Reaction Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. This process continues until stoichiometrically equivalent amounts of the reactants. Titration Basic Reaction.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry Titration Basic Reaction Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. Titration is a procedure used. Titration Basic Reaction.
From www.slideserve.com
PPT Ch 16 Redox Titrations PowerPoint Presentation ID6602548 Titration Basic Reaction Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. The basic process involves adding a. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A chemical reaction is set up between a known volume of. Titration Basic Reaction.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Titration Basic Reaction The basic process involves adding a. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In an indicator based titration you add another chemical that changes. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the. Titration Basic Reaction.
From mavink.com
Titration Reaction Titration Basic Reaction There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes. The basic process involves adding a. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been. A titration is a laboratory technique used to. Titration Basic Reaction.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID431911 Titration Basic Reaction Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known. Titration Basic Reaction.