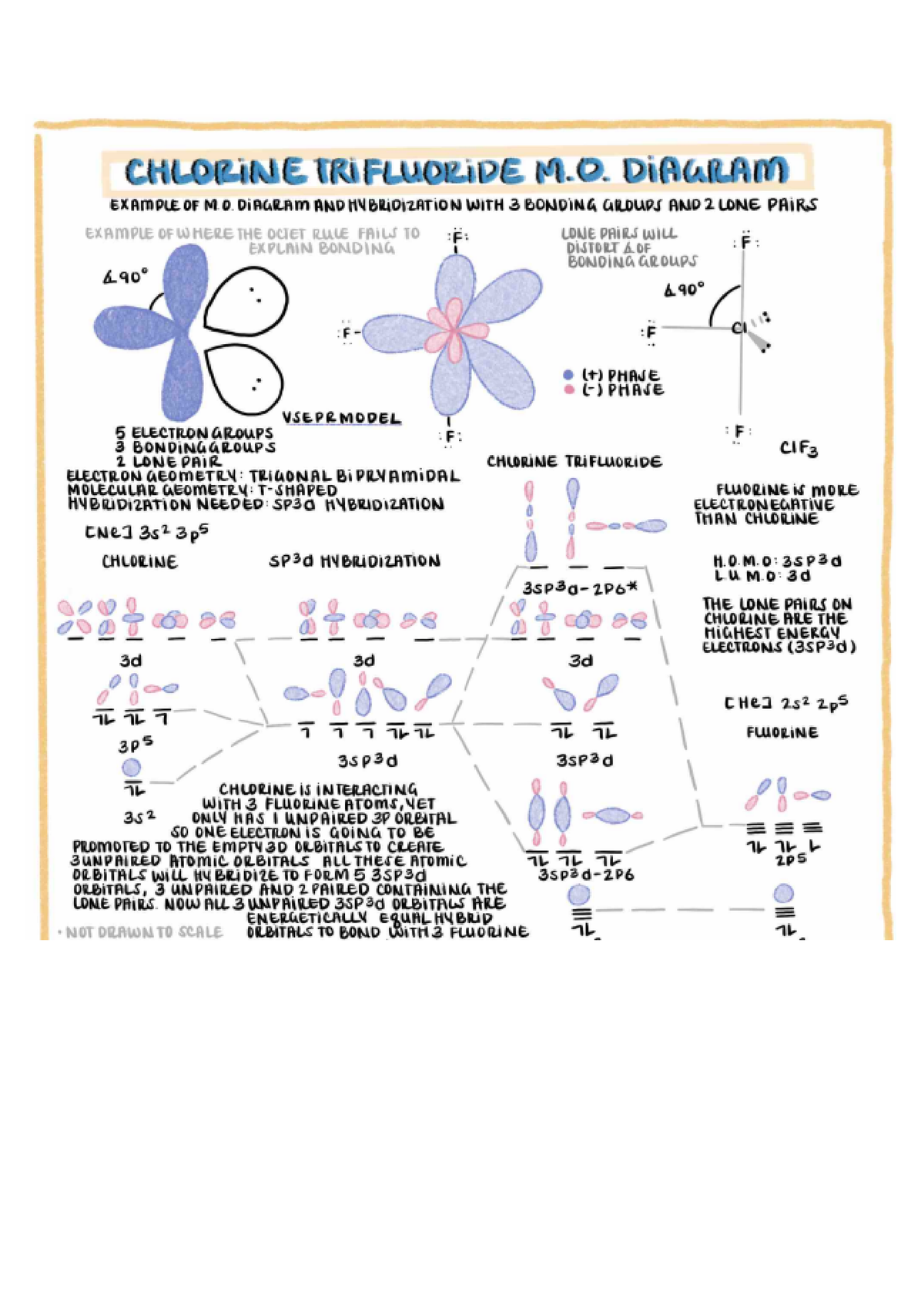
Chlorine Trifluoride Bond Length . Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom. There are two lone pairs on the central chlorine atom. Chlorine also forms covalent bonds with the surrounding fluorine atoms. In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. In addition, there are two lone pairs of electrons on the chlorine. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; One of the trigonal positions is occupied by the.
from www.studocu.com
There are two lone pairs on the central chlorine atom. One of the trigonal positions is occupied by the. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. In addition, there are two lone pairs of electrons on the chlorine. This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; Chlorine also forms covalent bonds with the surrounding fluorine atoms. To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom.
Chlorine Trifluoride M.O. Diagram CHEM12A Studocu
Chlorine Trifluoride Bond Length This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; One of the trigonal positions is occupied by the. Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom. This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. Chlorine also forms covalent bonds with the surrounding fluorine atoms. There are two lone pairs on the central chlorine atom. In addition, there are two lone pairs of electrons on the chlorine.
From www.bigstockphoto.com
Chlorine Trifluoride Image & Photo (Free Trial) Bigstock Chlorine Trifluoride Bond Length In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. There are two lone pairs on the central chlorine atom. To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; In addition, there are two lone pairs of electrons on. Chlorine Trifluoride Bond Length.
From www.studocu.com
Chlorine Trifluoride M.O. Diagram CHEM12A Studocu Chlorine Trifluoride Bond Length This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. In addition, there are two lone pairs of electrons on the chlorine. Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom.. Chlorine Trifluoride Bond Length.
From www.youtube.com
ClF3 (Chlorine trifluoride) Molecular Geometry, Bond Angles,& Electron Geometry YouTube Chlorine Trifluoride Bond Length This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Chlorine also forms covalent bonds with the surrounding fluorine atoms. In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. One of the trigonal positions is. Chlorine Trifluoride Bond Length.
From www.numerade.com
SOLVED 5) Chlorine trifluoride has two sets of lone pairs of electrons. Which position should Chlorine Trifluoride Bond Length In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. There are two lone pairs on the central chlorine atom. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. One of the trigonal positions is occupied by the. Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by. Chlorine Trifluoride Bond Length.
From www.youtube.com
ClF3 Molecular Geometry, Bond Angles & Electron Geometry (Chlorine Trifluoride) YouTube Chlorine Trifluoride Bond Length To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom. In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. There are two lone pairs on. Chlorine Trifluoride Bond Length.
From www.youtube.com
ClF3 Chlorine Trifluoride Shape Hybridisation VSEPR Problem Question Solved Chlorine Trifluoride Bond Length The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Chlorine also forms covalent bonds with the surrounding fluorine atoms. One of the trigonal positions is occupied by the. Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom. In $\ce{clf3}$ the central chlorine atom. Chlorine Trifluoride Bond Length.
From www.researchgate.net
Surface chemical process of silicon carbide caused by chlorine... Download Scientific Diagram Chlorine Trifluoride Bond Length The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. One of the trigonal positions is occupied by the. Chlorine also forms covalent bonds with the surrounding fluorine atoms. In $\ce{clf3}$ the. Chlorine Trifluoride Bond Length.
From www.numerade.com
SOLVED 5) Chlorine trifluoride has two sets of lone pairs of electrons. Which position should Chlorine Trifluoride Bond Length There are two lone pairs on the central chlorine atom. One of the trigonal positions is occupied by the. To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. Chlorine also forms covalent bonds with the surrounding fluorine atoms. In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. The lewis structure of. Chlorine Trifluoride Bond Length.
From www.studocu.com
Chemistry 101 Chapter 7 Part 3 Draw the Lewis structure of ClF₃, chlorine trifluoride Chlorine Trifluoride Bond Length This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom. To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. There are two lone pairs on the central chlorine atom. In $\ce{clf3}$ the central chlorine atom. Chlorine Trifluoride Bond Length.
From www.numerade.com
SOLVEDChlorine trifluoride, CIF3, is one of the most reactive compounds known. Write the Lewis Chlorine Trifluoride Bond Length There are two lone pairs on the central chlorine atom. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom. In addition, there are two lone pairs of electrons on the chlorine. To determine the. Chlorine Trifluoride Bond Length.
From www.ck12.org
Molecular Geometry CK12 Foundation Chlorine Trifluoride Bond Length There are two lone pairs on the central chlorine atom. In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. In addition, there are two lone pairs of electrons on the chlorine. To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. This means that we will have 3 $\ce{sp^2}$ orbitals emanating from. Chlorine Trifluoride Bond Length.
From www.bigstockphoto.com
Chlorine Trifluoride Image & Photo (Free Trial) Bigstock Chlorine Trifluoride Bond Length Chlorine also forms covalent bonds with the surrounding fluorine atoms. There are two lone pairs on the central chlorine atom. Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. To determine the hybridization, we. Chlorine Trifluoride Bond Length.
From www.istockphoto.com
Chlorine Trifluoride Molecular Structure Isolated On White Stock Photo Download Image Now iStock Chlorine Trifluoride Bond Length In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. In addition, there are two lone pairs of electrons on the chlorine. Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. There are two. Chlorine Trifluoride Bond Length.
From www.istockphoto.com
Chlorine Trifluoride Molecular Structure Isolated On White Stock Photo Download Image Now iStock Chlorine Trifluoride Bond Length There are two lone pairs on the central chlorine atom. One of the trigonal positions is occupied by the. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. In addition, there are two lone pairs of electrons on the chlorine. Chlorine trifluoride comprises 3 fluorine atoms, all pulled. Chlorine Trifluoride Bond Length.
From de-academic.com
Chlor(III)fluorid Chlorine Trifluoride Bond Length In addition, there are two lone pairs of electrons on the chlorine. In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom. There are two lone pairs on the central chlorine atom. One of the trigonal positions is occupied by the. Chlorine also forms covalent bonds. Chlorine Trifluoride Bond Length.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3. It is colourless Chlorine Trifluoride Bond Length One of the trigonal positions is occupied by the. To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. In addition, there are two lone pairs of electrons on the chlorine. There are two lone pairs on the central chlorine atom. Chlorine also forms covalent bonds with the surrounding fluorine atoms. In $\ce{clf3}$. Chlorine Trifluoride Bond Length.
From www.bigstockphoto.com
Chlorine Trifluoride Image & Photo (Free Trial) Bigstock Chlorine Trifluoride Bond Length Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom. This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. One of the trigonal positions is occupied by the. In $\ce{clf3}$ the. Chlorine Trifluoride Bond Length.
From chemicalportal.ru
Фторид хлора (III) — свойства, получение и применение Chlorine Trifluoride Bond Length This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. Chlorine also forms covalent bonds with the surrounding fluorine atoms. There are two lone pairs on the central chlorine atom. Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by. Chlorine Trifluoride Bond Length.
From www.shutterstock.com
Chlorine Trifluoride Molecular Structure Formula Periodic Stock Vector (Royalty Free) 2312393245 Chlorine Trifluoride Bond Length This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Chlorine also forms covalent bonds with the surrounding fluorine atoms. In addition, there are two lone. Chlorine Trifluoride Bond Length.
From brainly.in
Draw structure of chlorine trifluoride Brainly.in Chlorine Trifluoride Bond Length This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; Chlorine also forms covalent bonds with the surrounding fluorine atoms. There are two lone pairs on the central chlorine atom. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Chlorine trifluoride comprises 3 fluorine atoms,. Chlorine Trifluoride Bond Length.
From slideplayer.com
Molecular Geometry. ppt download Chlorine Trifluoride Bond Length To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. There are two lone pairs on the central chlorine atom. One of the trigonal positions is occupied by the. Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom.. Chlorine Trifluoride Bond Length.
From www.numerade.com
SOLVED Draw Lewis structures for each molecule chlorine trifluoride, ClF3 nitrogen trifluoride Chlorine Trifluoride Bond Length The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Chlorine also forms covalent bonds with the surrounding fluorine atoms. Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom. This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; To determine. Chlorine Trifluoride Bond Length.
From interestingengineering.com
Chlorine Trifluoride The Compound That Can Even Set Fire to Glass Chlorine Trifluoride Bond Length Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom. Chlorine also forms covalent bonds with the surrounding fluorine atoms. In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; In addition, there are two lone pairs of electrons on the chlorine. The. Chlorine Trifluoride Bond Length.
From animalia-life.club
Bond Length Periodic Table Chlorine Trifluoride Bond Length This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom. One of the trigonal positions is occupied by the. In addition, there are two lone pairs. Chlorine Trifluoride Bond Length.
From www.slideserve.com
PPT VSEPR. PowerPoint Presentation ID214953 Chlorine Trifluoride Bond Length Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom. In addition, there are two lone pairs of electrons on the chlorine. Chlorine also forms covalent bonds with the surrounding fluorine atoms. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. There are two. Chlorine Trifluoride Bond Length.
From readingandwritingprojectcom.web.fc2.com
what is the electronic geometry of clf3 Chlorine Trifluoride Bond Length To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; Chlorine also forms covalent bonds with the surrounding fluorine atoms. One of the trigonal positions is occupied by the. The. Chlorine Trifluoride Bond Length.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3. It is colourless Chlorine Trifluoride Bond Length Chlorine also forms covalent bonds with the surrounding fluorine atoms. There are two lone pairs on the central chlorine atom. Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom. In addition, there are two lone pairs of electrons on the chlorine. This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; The. Chlorine Trifluoride Bond Length.
From www.pngegg.com
Free download Chlorine trifluoride Sodium molybdate NFPA 704 Etching, Chlorine Trifluoride Chlorine Trifluoride Bond Length To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. In addition, there are two lone pairs of electrons on the chlorine. Chlorine also forms covalent bonds with the surrounding fluorine atoms. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms.. Chlorine Trifluoride Bond Length.
From www.jobilize.com
Fluorides of chlorine, Compounds of chlorine, By OpenStax (Page 2/2) Jobilize Chlorine Trifluoride Bond Length There are two lone pairs on the central chlorine atom. Chlorine trifluoride comprises 3 fluorine atoms, all pulled together by the central chlorine atom. In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. One of the trigonal positions is occupied by the. Chlorine also forms covalent bonds with the surrounding fluorine atoms. In addition, there are two lone pairs. Chlorine Trifluoride Bond Length.
From imgbin.com
Nitrogen Trifluoride Molecule Ballandstick Model Trigonal Pyramidal Molecular Geometry PNG Chlorine Trifluoride Bond Length In addition, there are two lone pairs of electrons on the chlorine. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Chlorine also forms covalent bonds with the surrounding fluorine atoms. There are two lone pairs on the central chlorine atom. This means that we will have 3. Chlorine Trifluoride Bond Length.
From www.anyrgb.com
Binary Phase, Xenon difluoride, Xenon tetrafluoride, chlorine Trifluoride, geometri, Lone pair Chlorine Trifluoride Bond Length The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. Chlorine also forms covalent bonds with the surrounding fluorine atoms. One of the trigonal positions is occupied by the. To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. Chlorine trifluoride comprises. Chlorine Trifluoride Bond Length.
From valenceelectrons.com
How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)? Chlorine Trifluoride Bond Length In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. In addition, there are two lone pairs of electrons on the chlorine. Chlorine also forms covalent bonds with the surrounding fluorine atoms. One of the trigonal positions is occupied by the. The lewis. Chlorine Trifluoride Bond Length.
From www.youtube.com
Molecular Structure of Chlorine Trifluoride Using VSEPR Theory Nature of Chemical Bond YouTube Chlorine Trifluoride Bond Length One of the trigonal positions is occupied by the. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at the center bonded to three fluorine atoms. This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the chlorine; To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule.. Chlorine Trifluoride Bond Length.
From www.bigstockphoto.com
Chlorine Trifluoride Image & Photo (Free Trial) Bigstock Chlorine Trifluoride Bond Length In addition, there are two lone pairs of electrons on the chlorine. To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. One of the trigonal positions is occupied by the. There are two lone pairs on the central chlorine atom. This means that we will have 3 $\ce{sp^2}$ orbitals emanating from the. Chlorine Trifluoride Bond Length.
From www.numerade.com
SOLVED Gaseous chlorine trifluoride is a compound used in the production of nuclear fuels. It Chlorine Trifluoride Bond Length One of the trigonal positions is occupied by the. There are two lone pairs on the central chlorine atom. To determine the hybridization, we take a look at the lewis structure of the clf 3 molecule. In $\ce{clf3}$ the central chlorine atom is roughly $\ce{sp^2}$ hybridized. The lewis structure of clf3, or chlorine trifluoride, consists of a chlorine atom at. Chlorine Trifluoride Bond Length.