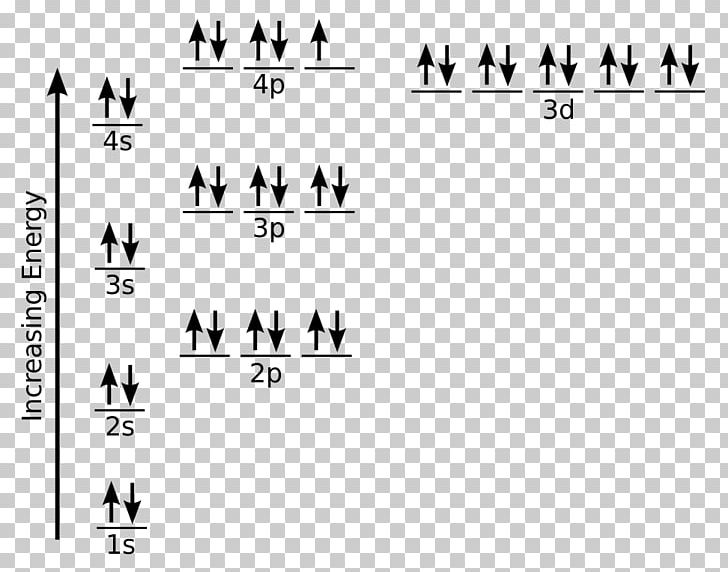
Fe Electron Configuration Orbital Diagram . Fe, or iron, is a transition metal with atomic number 26. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. The orbital diagram for fe can be determined by following. Iron electron configuration (fe) or ferric electron configuration with orbital diagram have been provided here with complete information. Electron configurations and orbital box diagrams can be written right from the periodic table. It has a unique electron configuration due to its location in the periodic table. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. The initial two electrons in the electron configuration for iron will be in the 1s orbital. To write the orbital diagram for the iron (fe) first we need to write the electron configuration for.
from modernizemodest1712.blogspot.com
The initial two electrons in the electron configuration for iron will be in the 1s orbital. Fe, or iron, is a transition metal with atomic number 26. Electron configurations and orbital box diagrams can be written right from the periodic table. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. To write the orbital diagram for the iron (fe) first we need to write the electron configuration for. Iron electron configuration (fe) or ferric electron configuration with orbital diagram have been provided here with complete information. The orbital diagram for fe can be determined by following. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. It has a unique electron configuration due to its location in the periodic table.
40 orbital diagram for fe Diagram For You
Fe Electron Configuration Orbital Diagram It has a unique electron configuration due to its location in the periodic table. Fe, or iron, is a transition metal with atomic number 26. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. Electron configurations and orbital box diagrams can be written right from the periodic table. Iron electron configuration (fe) or ferric electron configuration with orbital diagram have been provided here with complete information. To write the orbital diagram for the iron (fe) first we need to write the electron configuration for. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. It has a unique electron configuration due to its location in the periodic table. The initial two electrons in the electron configuration for iron will be in the 1s orbital. The orbital diagram for fe can be determined by following.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Fe Electron Configuration Orbital Diagram Electron configurations and orbital box diagrams can be written right from the periodic table. The orbital diagram for fe can be determined by following. Fe, or iron, is a transition metal with atomic number 26. The initial two electrons in the electron configuration for iron will be in the 1s orbital. Because the 1s orbital can only hold two electrons,. Fe Electron Configuration Orbital Diagram.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Fe Electron Configuration Orbital Diagram Fe, or iron, is a transition metal with atomic number 26. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. Electron configurations and orbital box diagrams can be written right from the periodic table. It has a unique electron configuration due to its location in the periodic table. The orbital diagram for. Fe Electron Configuration Orbital Diagram.
From www.numerade.com
SOLVED18. Write out the electron configuration for Fe" , Fe'? and Fe"' , Show the orbitals for Fe Electron Configuration Orbital Diagram Electron configurations and orbital box diagrams can be written right from the periodic table. The initial two electrons in the electron configuration for iron will be in the 1s orbital. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. Fe,. Fe Electron Configuration Orbital Diagram.
From www.slideserve.com
PPT Orbital Diagrams and Electron Configuration PowerPoint Presentation ID726352 Fe Electron Configuration Orbital Diagram Iron electron configuration (fe) or ferric electron configuration with orbital diagram have been provided here with complete information. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. Because the 1s orbital can only hold two electrons, iron’s next two electrons. Fe Electron Configuration Orbital Diagram.
From mavink.com
Electron Orbital Configuration Chart Fe Electron Configuration Orbital Diagram Fe, or iron, is a transition metal with atomic number 26. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. The orbital diagram for fe can be determined by following. The initial two electrons in the electron configuration for iron will be in the 1s orbital. Iron electron configuration (fe) or ferric. Fe Electron Configuration Orbital Diagram.
From valenceelectrons.com
Iron(Fe) electron configuration and orbital diagram Fe Electron Configuration Orbital Diagram The initial two electrons in the electron configuration for iron will be in the 1s orbital. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on. Fe Electron Configuration Orbital Diagram.
From pt.slideshare.net
Electron configuration Fe Electron Configuration Orbital Diagram Electron configurations and orbital box diagrams can be written right from the periodic table. It has a unique electron configuration due to its location in the periodic table. Fe, or iron, is a transition metal with atomic number 26. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the. Fe Electron Configuration Orbital Diagram.
From stewart-switch.com
Fe 3+ Orbital Diagram Fe Electron Configuration Orbital Diagram Fe, or iron, is a transition metal with atomic number 26. Iron electron configuration (fe) or ferric electron configuration with orbital diagram have been provided here with complete information. To write the orbital diagram for the iron (fe) first we need to write the electron configuration for. It has a unique electron configuration due to its location in the periodic. Fe Electron Configuration Orbital Diagram.
From www.alamy.com
Iron (Fe). Diagram of the nuclear composition, electron configuration, chemical data, and Fe Electron Configuration Orbital Diagram The orbital diagram for fe can be determined by following. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. Iron electron configuration (fe) or ferric electron configuration with orbital diagram have been provided here with complete information. An orbital diagram, like those shown above, is a visual way to reconstruct the electron. Fe Electron Configuration Orbital Diagram.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Fe Electron Configuration Orbital Diagram An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. It has a unique electron configuration due to its location in the periodic table. Electron configurations and orbital box diagrams can be written right from the periodic table. The orbital diagram. Fe Electron Configuration Orbital Diagram.
From opentextbc.ca
Organization of Electrons in Atoms Introductory Chemistry 1st Canadian Edition Fe Electron Configuration Orbital Diagram The initial two electrons in the electron configuration for iron will be in the 1s orbital. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. The orbital diagram for fe can be determined by following. Electron configurations and orbital box diagrams can be written right from the periodic table. An orbital diagram,. Fe Electron Configuration Orbital Diagram.
From quizlet.com
Draw the electron configuration for a neutral atom of iron. Quizlet Fe Electron Configuration Orbital Diagram Electron configurations and orbital box diagrams can be written right from the periodic table. Fe, or iron, is a transition metal with atomic number 26. The initial two electrons in the electron configuration for iron will be in the 1s orbital. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. To write. Fe Electron Configuration Orbital Diagram.
From www.chem.fsu.edu
Electron Configurations Fe Electron Configuration Orbital Diagram Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. Iron electron configuration (fe) or ferric electron configuration with orbital diagram have been provided here with complete information. To write the orbital diagram for the iron (fe) first we need to write the electron configuration for. It has a unique electron configuration due. Fe Electron Configuration Orbital Diagram.
From wirepartcavalierly.z21.web.core.windows.net
Determine The Electron Configuration Of Fe3+ Fe Electron Configuration Orbital Diagram Fe, or iron, is a transition metal with atomic number 26. Iron electron configuration (fe) or ferric electron configuration with orbital diagram have been provided here with complete information. The initial two electrons in the electron configuration for iron will be in the 1s orbital. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned. Fe Electron Configuration Orbital Diagram.
From manuallistcantabank.z21.web.core.windows.net
Chemistry Orbital Diagram Fe Electron Configuration Orbital Diagram Fe, or iron, is a transition metal with atomic number 26. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. The initial two electrons in the electron configuration for iron will be in the 1s orbital. Electron configurations and orbital box diagrams can be written right from the periodic table. It has. Fe Electron Configuration Orbital Diagram.
From modernizemodest1712.blogspot.com
40 orbital diagram for fe Diagram For You Fe Electron Configuration Orbital Diagram Electron configurations and orbital box diagrams can be written right from the periodic table. To write the orbital diagram for the iron (fe) first we need to write the electron configuration for. The orbital diagram for fe can be determined by following. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. An. Fe Electron Configuration Orbital Diagram.
From wisc.pb.unizin.org
Electron Configurations, Orbital Box Notation (M7Q7) UWMadison Chemistry 103/104 Resource Book Fe Electron Configuration Orbital Diagram Iron electron configuration (fe) or ferric electron configuration with orbital diagram have been provided here with complete information. The initial two electrons in the electron configuration for iron will be in the 1s orbital. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. It has a unique electron configuration due to its. Fe Electron Configuration Orbital Diagram.
From modernizemodest1712.blogspot.com
40 orbital diagram for fe Diagram For You Fe Electron Configuration Orbital Diagram An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. The orbital diagram for fe can be determined by following. Iron electron configuration (fe) or ferric electron configuration with orbital diagram have been provided here with complete information. Fe, or iron,. Fe Electron Configuration Orbital Diagram.
From modernizemodest1712.blogspot.com
40 orbital diagram for fe Diagram For You Fe Electron Configuration Orbital Diagram An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. To write the orbital diagram for the iron (fe) first we need to write the electron configuration for. It has a unique electron configuration due to its location in the periodic. Fe Electron Configuration Orbital Diagram.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Fe Electron Configuration Orbital Diagram The initial two electrons in the electron configuration for iron will be in the 1s orbital. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. Fe, or iron, is a transition metal with atomic number 26. To write the orbital. Fe Electron Configuration Orbital Diagram.
From modernizemodest1712.blogspot.com
40 orbital diagram for fe Diagram For You Fe Electron Configuration Orbital Diagram The initial two electrons in the electron configuration for iron will be in the 1s orbital. The orbital diagram for fe can be determined by following. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. It has a unique electron configuration due to its location in the periodic table. An orbital diagram,. Fe Electron Configuration Orbital Diagram.
From galvinconanstuart.blogspot.com
Fe Orbital Diagram General Wiring Diagram Fe Electron Configuration Orbital Diagram Electron configurations and orbital box diagrams can be written right from the periodic table. Fe, or iron, is a transition metal with atomic number 26. The initial two electrons in the electron configuration for iron will be in the 1s orbital. The orbital diagram for fe can be determined by following. It has a unique electron configuration due to its. Fe Electron Configuration Orbital Diagram.
From chem.libretexts.org
Chapter 2.7 Electronic Structure of the Transition Metals Chemistry LibreTexts Fe Electron Configuration Orbital Diagram It has a unique electron configuration due to its location in the periodic table. To write the orbital diagram for the iron (fe) first we need to write the electron configuration for. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the. Fe Electron Configuration Orbital Diagram.
From modernizemodest1712.blogspot.com
40 orbital diagram for fe Diagram For You Fe Electron Configuration Orbital Diagram The orbital diagram for fe can be determined by following. Electron configurations and orbital box diagrams can be written right from the periodic table. The initial two electrons in the electron configuration for iron will be in the 1s orbital. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of. Fe Electron Configuration Orbital Diagram.
From manuallistcantabank.z21.web.core.windows.net
Electron Configuration Orbital Diagram Fe Electron Configuration Orbital Diagram The orbital diagram for fe can be determined by following. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. Electron configurations and orbital box diagrams can be written right from the periodic table. It has a unique electron configuration due to its location in the periodic table. Iron electron configuration (fe) or. Fe Electron Configuration Orbital Diagram.
From www.youtube.com
Iron (Fe) Electron Configuration YouTube Fe Electron Configuration Orbital Diagram Iron electron configuration (fe) or ferric electron configuration with orbital diagram have been provided here with complete information. It has a unique electron configuration due to its location in the periodic table. The orbital diagram for fe can be determined by following. The initial two electrons in the electron configuration for iron will be in the 1s orbital. An orbital. Fe Electron Configuration Orbital Diagram.
From ibmole.com
2 +12. Atomic structure Fe Electron Configuration Orbital Diagram To write the orbital diagram for the iron (fe) first we need to write the electron configuration for. Fe, or iron, is a transition metal with atomic number 26. The initial two electrons in the electron configuration for iron will be in the 1s orbital. It has a unique electron configuration due to its location in the periodic table. Electron. Fe Electron Configuration Orbital Diagram.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Fe Electron Configuration Orbital Diagram Electron configurations and orbital box diagrams can be written right from the periodic table. To write the orbital diagram for the iron (fe) first we need to write the electron configuration for. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the. Fe Electron Configuration Orbital Diagram.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts Fe Electron Configuration Orbital Diagram Electron configurations and orbital box diagrams can be written right from the periodic table. Fe, or iron, is a transition metal with atomic number 26. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. The orbital diagram for fe can. Fe Electron Configuration Orbital Diagram.
From ar.inspiredpencil.com
Iron Orbital Notation Fe Electron Configuration Orbital Diagram Electron configurations and orbital box diagrams can be written right from the periodic table. Fe, or iron, is a transition metal with atomic number 26. To write the orbital diagram for the iron (fe) first we need to write the electron configuration for. The orbital diagram for fe can be determined by following. Iron electron configuration (fe) or ferric electron. Fe Electron Configuration Orbital Diagram.
From www.youtube.com
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions) YouTube Fe Electron Configuration Orbital Diagram It has a unique electron configuration due to its location in the periodic table. Iron electron configuration (fe) or ferric electron configuration with orbital diagram have been provided here with complete information. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. The orbital diagram for fe can be determined by following. The. Fe Electron Configuration Orbital Diagram.
From socratic.org
What is the shape of forbital??? + Example Fe Electron Configuration Orbital Diagram Fe, or iron, is a transition metal with atomic number 26. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. The initial two electrons in the electron configuration for iron will be in the 1s orbital. It has a unique. Fe Electron Configuration Orbital Diagram.
From ar.inspiredpencil.com
Electron Configuration Diagram Orbitals Fe Electron Configuration Orbital Diagram Electron configurations and orbital box diagrams can be written right from the periodic table. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons.. Fe Electron Configuration Orbital Diagram.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Fe Electron Configuration Orbital Diagram Fe, or iron, is a transition metal with atomic number 26. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. Iron electron configuration (fe) or ferric electron configuration with orbital diagram have been provided here with complete information. The initial. Fe Electron Configuration Orbital Diagram.
From newlasertagatlanta.blogspot.com
42 fe2+ orbital diagram Wiring Diagrams Manual Fe Electron Configuration Orbital Diagram The initial two electrons in the electron configuration for iron will be in the 1s orbital. Iron electron configuration (fe) or ferric electron configuration with orbital diagram have been provided here with complete information. Because the 1s orbital can only hold two electrons, iron’s next two electrons are assigned to the. Electron configurations and orbital box diagrams can be written. Fe Electron Configuration Orbital Diagram.