Common Indicators Used In Titration . Any of the three indicators will exhibit. When choosing the appropriate indicator, the ph of the equivalence point is very. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their. This page assumes that you know about ph curves for all the. Both indicators change colour over a specific ph range. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Indicators are substances that change colour when they are added to acidic or alkaline solutions; The two most common indicators that are used in titrations are methyl orange and phenolphthalein.
from slidetodoc.com
This page assumes that you know about ph curves for all the. Both indicators change colour over a specific ph range. Indicators are substances that change colour when they are added to acidic or alkaline solutions; When choosing the appropriate indicator, the ph of the equivalence point is very. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their. Any of the three indicators will exhibit.
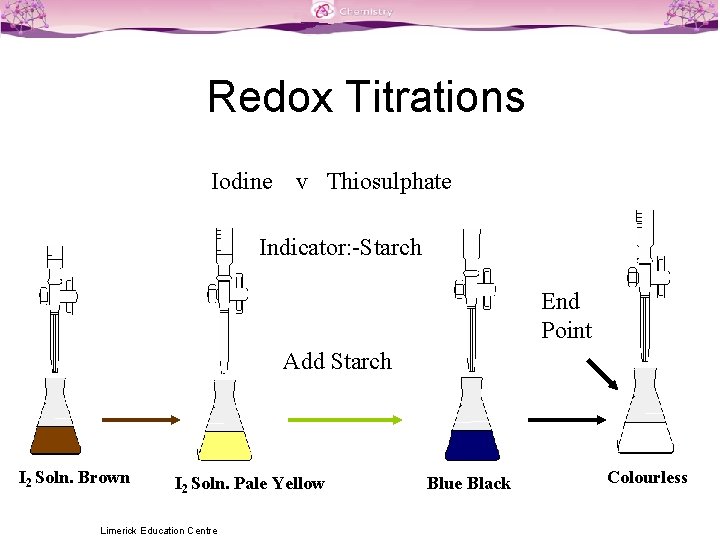
Titration Colour Changes SLSS Science Limerick Education Centre
Common Indicators Used In Titration Both indicators change colour over a specific ph range. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. When choosing the appropriate indicator, the ph of the equivalence point is very. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Any of the three indicators will exhibit. Both indicators change colour over a specific ph range. Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know about ph curves for all the. They are usually weak acids or bases, but their. Indicators are substances that change colour when they are added to acidic or alkaline solutions;
From hxeaejnht.blob.core.windows.net
Base Titration Indicator Used at Felicia Sullivan blog Common Indicators Used In Titration Any of the three indicators will exhibit. This page assumes that you know about ph curves for all the. Indicators are substances that change colour when they are added to acidic or alkaline solutions; The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. They are usually weak acids or bases, but their. Indicators are. Common Indicators Used In Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Common Indicators Used In Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Any of the three indicators will exhibit. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Indicators are substances that change colour when they are added to acidic or alkaline solutions; They are usually weak acids or bases, but. Common Indicators Used In Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Common Indicators Used In Titration They are usually weak acids or bases, but their. Indicators are substances that change colour when they are added to acidic or alkaline solutions; The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Any of the three indicators will exhibit. The two most common indicators that are used in titrations are methyl orange and. Common Indicators Used In Titration.
From gioteyspe.blob.core.windows.net
Indicator Used For Complexometric Titrations at Jerlene Powell blog Common Indicators Used In Titration This page assumes that you know about ph curves for all the. Any of the three indicators will exhibit. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Indicators are substances that change colour when they are added to. Common Indicators Used In Titration.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations (GCSE) — the science hive Common Indicators Used In Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Indicators are substances whose solutions change color due to changes in ph. Both indicators change colour over a specific ph range. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. When choosing the appropriate indicator, the ph of the. Common Indicators Used In Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Common Indicators Used In Titration They are usually weak acids or bases, but their. Indicators are substances whose solutions change color due to changes in ph. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Indicators are substances that change colour when they are. Common Indicators Used In Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Common Indicators Used In Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Indicators are substances that change colour when they are added to acidic or alkaline solutions; They are usually weak acids or bases, but their. Both indicators change colour over a. Common Indicators Used In Titration.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Common Indicators Used In Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. This page assumes that you know about ph curves for all the. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their. The graph shows the results obtained using two indicators (methyl red and. Common Indicators Used In Titration.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Common Indicators Used In Titration They are usually weak acids or bases, but their. Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know about ph curves for all the. Any of the three indicators will exhibit. Indicators are substances that change colour when they are added to acidic or alkaline solutions; The graph shows the results. Common Indicators Used In Titration.
From exogrvkja.blob.core.windows.net
Indicators For Titrations at Leslie Jackson blog Common Indicators Used In Titration This page assumes that you know about ph curves for all the. Both indicators change colour over a specific ph range. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. When choosing the appropriate indicator, the ph of the equivalence point is very. The graph shows the results obtained using two indicators (methyl red. Common Indicators Used In Titration.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID5572517 Common Indicators Used In Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. This page assumes that you know about ph curves for all the. They are usually weak acids or bases, but their. Any of the three indicators will exhibit. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators. Common Indicators Used In Titration.
From mungfali.com
Acid Base Titration Indicator Common Indicators Used In Titration They are usually weak acids or bases, but their. When choosing the appropriate indicator, the ph of the equivalence point is very. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. This page assumes that you know about ph curves for all the. Any of the three indicators will exhibit. Both indicators change colour. Common Indicators Used In Titration.
From courses.lumenlearning.com
AcidBase Titrations Boundless Chemistry Common Indicators Used In Titration They are usually weak acids or bases, but their. Indicators are substances whose solutions change color due to changes in ph. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Any of the three indicators will exhibit. Indicators are substances that change colour when they are added to acidic or alkaline solutions; When choosing. Common Indicators Used In Titration.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Common Indicators Used In Titration This page assumes that you know about ph curves for all the. Indicators are substances that change colour when they are added to acidic or alkaline solutions; The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators change. Common Indicators Used In Titration.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Common Indicators Used In Titration Any of the three indicators will exhibit. When choosing the appropriate indicator, the ph of the equivalence point is very. Indicators are substances that change colour when they are added to acidic or alkaline solutions; The two most common indicators that are used in titrations are methyl orange and phenolphthalein. They are usually weak acids or bases, but their. This. Common Indicators Used In Titration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Common Indicators Used In Titration Indicators are substances whose solutions change color due to changes in ph. Both indicators change colour over a specific ph range. Any of the three indicators will exhibit. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. When choosing. Common Indicators Used In Titration.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Common Indicators Used In Titration Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know about ph curves for all the. They are usually weak acids or bases, but their. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Indicators are substances that change colour when they are added to acidic. Common Indicators Used In Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Common Indicators Used In Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. When choosing the appropriate indicator, the ph of the equivalence point is very. Indicators are substances whose solutions change color due to changes in ph. This page assumes that you. Common Indicators Used In Titration.
From gioteyspe.blob.core.windows.net
Indicator Used For Complexometric Titrations at Jerlene Powell blog Common Indicators Used In Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. This page assumes that you know about ph curves for all the. When choosing the appropriate indicator, the ph of the equivalence point is very. Any of the three indicators will exhibit. Both indicators change colour over a specific ph range. They are usually weak. Common Indicators Used In Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Common Indicators Used In Titration When choosing the appropriate indicator, the ph of the equivalence point is very. Indicators are substances that change colour when they are added to acidic or alkaline solutions; They are usually weak acids or bases, but their. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Any of the three indicators will exhibit. Indicators. Common Indicators Used In Titration.
From www.vrogue.co
Titration Indicator Types Procedure Indicators vrogue.co Common Indicators Used In Titration They are usually weak acids or bases, but their. Both indicators change colour over a specific ph range. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Any of the three indicators will exhibit. Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know about ph. Common Indicators Used In Titration.
From studylib.net
The titration of an Indicator (HIn) Common Indicators Used In Titration This page assumes that you know about ph curves for all the. They are usually weak acids or bases, but their. Indicators are substances whose solutions change color due to changes in ph. Both indicators change colour over a specific ph range. Any of the three indicators will exhibit. Indicators are substances that change colour when they are added to. Common Indicators Used In Titration.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Common Indicators Used In Titration Any of the three indicators will exhibit. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Indicators are substances that change colour when they are added to acidic or alkaline solutions; Indicators are substances whose solutions change color due to changes in ph. The graph shows the results obtained using two indicators (methyl red. Common Indicators Used In Titration.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.7.13 Indicators used in Titration翰林国际教育 Common Indicators Used In Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators change colour over a specific ph range. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. This page assumes that you know about ph curves for all the. They are usually weak acids or bases, but their.. Common Indicators Used In Titration.
From www.vrogue.co
Titration Indicator Types Procedure Indicators vrogue.co Common Indicators Used In Titration Indicators are substances whose solutions change color due to changes in ph. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. When choosing the appropriate indicator, the ph of the equivalence point is very. This page assumes that you. Common Indicators Used In Titration.
From www.slideserve.com
PPT Indicators for AcidBase Titrations (Sec. 96) PowerPoint Common Indicators Used In Titration Indicators are substances that change colour when they are added to acidic or alkaline solutions; Indicators are substances whose solutions change color due to changes in ph. Both indicators change colour over a specific ph range. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Any of the three indicators will exhibit. When choosing. Common Indicators Used In Titration.
From www.researchgate.net
Main features of common redox indicators and mediators used in Common Indicators Used In Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. This page assumes that you know about ph curves for all the. Both indicators change colour over a specific ph range. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Indicators are substances whose solutions change color due to. Common Indicators Used In Titration.
From www.slideshare.net
Acid base titration Common Indicators Used In Titration Indicators are substances that change colour when they are added to acidic or alkaline solutions; They are usually weak acids or bases, but their. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. When choosing the appropriate indicator, the ph of the equivalence point is very. Indicators are substances whose solutions change color due. Common Indicators Used In Titration.
From www.microlit.com
An Advanced Guide to Titration Microlit Common Indicators Used In Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. When choosing the appropriate indicator, the ph of the equivalence point is very. Indicators are substances that change colour when they are added to acidic or alkaline solutions; Indicators are substances whose solutions change color due to changes in ph. Any of the three indicators. Common Indicators Used In Titration.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Common Indicators Used In Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Any of the three indicators will exhibit. Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know about ph curves for all the. They are usually weak acids or bases, but their. When choosing the appropriate indicator,. Common Indicators Used In Titration.
From www.numerade.com
SOLVED Methyl orange and phenolpthalein are common chemical indicators Common Indicators Used In Titration Indicators are substances whose solutions change color due to changes in ph. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. This page assumes that you know about ph curves for all the. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. When choosing the appropriate indicator, the. Common Indicators Used In Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Common Indicators Used In Titration Any of the three indicators will exhibit. When choosing the appropriate indicator, the ph of the equivalence point is very. Indicators are substances whose solutions change color due to changes in ph. Indicators are substances that change colour when they are added to acidic or alkaline solutions; The two most common indicators that are used in titrations are methyl orange. Common Indicators Used In Titration.
From school.careers360.com
redox titration Overview, Structure, Properties & Uses Common Indicators Used In Titration Indicators are substances that change colour when they are added to acidic or alkaline solutions; Any of the three indicators will exhibit. This page assumes that you know about ph curves for all the. They are usually weak acids or bases, but their. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators. Common Indicators Used In Titration.
From slidetodoc.com
ACID BASE TITRATION INDICATORS Titration a method of Common Indicators Used In Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Both indicators change colour over a specific ph range. Any of the three indicators will exhibit. They are usually weak acids or bases, but their. Indicators are substances whose solutions change color due to changes in ph. When choosing the appropriate indicator, the ph of. Common Indicators Used In Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Common Indicators Used In Titration Both indicators change colour over a specific ph range. Any of the three indicators will exhibit. Indicators are substances that change colour when they are added to acidic or alkaline solutions; When choosing the appropriate indicator, the ph of the equivalence point is very. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. They. Common Indicators Used In Titration.