Iron Electron Configuration Full . Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Electron configuration chart of all elements is mentioned in the table below. Since 1s can only hold two electrons the next 2. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. In writing the electron configuration for iron the first two electrons will go in the 1s orbital. Iron is a transition metal with symbol fe and atomic number 26. The shorthand electron configuration (or noble gas configuration) as well as. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Its electron configuration is [ar] 3d 6 4s 2, which means it has two valence. It’s a transition metal from group 8 of the periodic table’s first transition series.
from www.sciencefacts.net
Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. Its electron configuration is [ar] 3d 6 4s 2, which means it has two valence. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Iron is a transition metal with symbol fe and atomic number 26. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Since 1s can only hold two electrons the next 2. In writing the electron configuration for iron the first two electrons will go in the 1s orbital. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and.
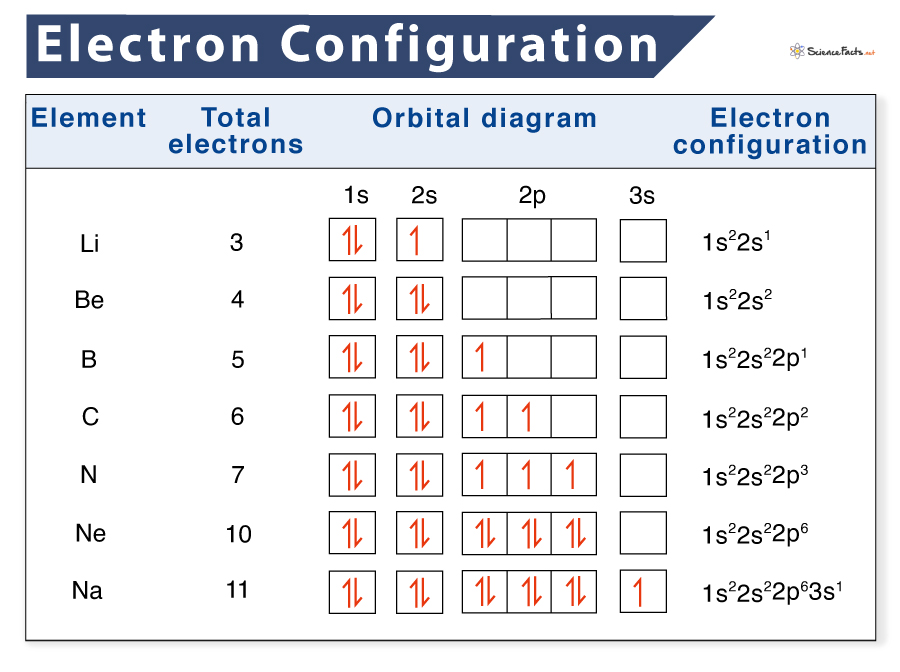
Electron Configuration Definition, Examples, Chart, and Diagram
Iron Electron Configuration Full In writing the electron configuration for iron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. In writing the electron configuration for iron the first two electrons will go in the 1s orbital. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. It’s a transition metal from group 8 of the periodic table’s first transition series. Its electron configuration is [ar] 3d 6 4s 2, which means it has two valence. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. Iron is a transition metal with symbol fe and atomic number 26. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. The chemical element iron has the atomic number 26 and the symbol fe (from latin:
From www.youtube.com
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions Iron Electron Configuration Full Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. It’s a transition metal from group 8 of the periodic table’s first transition series. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number. Iron Electron Configuration Full.
From ar.inspiredpencil.com
Iron Orbital Notation Iron Electron Configuration Full The chemical element iron has the atomic number 26 and the symbol fe (from latin: Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. The shorthand electron configuration (or noble gas configuration) as well as. Find the full electronic. Iron Electron Configuration Full.
From utedzz.blogspot.com
Periodic Table Iron Valence Electrons Periodic Table Timeline Iron Electron Configuration Full Since 1s can only hold two electrons the next 2. Iron is a transition metal with symbol fe and atomic number 26. Its electron configuration is [ar] 3d 6 4s 2, which means it has two valence. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. Iron is on the fourth row of the periodic table, sixth column. Iron Electron Configuration Full.
From ar.inspiredpencil.com
Iron Orbital Notation Iron Electron Configuration Full The chemical element iron has the atomic number 26 and the symbol fe (from latin: It’s a transition metal from group 8 of the periodic table’s first transition series. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The shorthand electron configuration (or noble gas configuration) as well as. Since 1s can. Iron Electron Configuration Full.
From www.alamy.com
Iron (Fe). Diagram of the nuclear composition and electron Iron Electron Configuration Full Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. It’s. Iron Electron Configuration Full.
From anelementaday.wordpress.com
Day 3 Iron An Element A Day Iron Electron Configuration Full Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. In writing the electron configuration for iron the first two electrons will go in the 1s orbital. Iron is a transition metal with symbol fe and atomic number 26. Electron configuration chart of all elements is mentioned in the table below.. Iron Electron Configuration Full.
From justanothershop-a-holic.blogspot.com
Electron Configuration Of Iron Full worksheet today Iron Electron Configuration Full The chemical element iron has the atomic number 26 and the symbol fe (from latin: It’s a transition metal from group 8 of the periodic table’s first transition series. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. The shorthand electron configuration (or noble gas configuration) as well as. Since 1s can only hold two electrons the next. Iron Electron Configuration Full.
From ar.inspiredpencil.com
Electron Configuration Of Iron Iron Electron Configuration Full It’s a transition metal from group 8 of the periodic table’s first transition series. In writing the electron configuration for iron the first two electrons will go in the 1s orbital. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Its electron configuration is [ar] 3d 6 4s 2, which means it has two. Iron Electron Configuration Full.
From www.youtube.com
Electron Configuration of Transition Metals Iron Fe Cobalt Co and Iron Electron Configuration Full Electron configuration chart of all elements is mentioned in the table below. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Its electron configuration is [ar] 3d 6 4s 2, which means it has two valence. The shorthand electron configuration (or noble gas configuration) as well as. Iron is a transition metal. Iron Electron Configuration Full.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Iron Electron Configuration Full Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. The chemical element iron has the atomic number 26 and the symbol fe (from latin: It’s a transition metal from group 8 of the periodic table’s first transition series. Electron configuration chart of all elements is mentioned in the table below.. Iron Electron Configuration Full.
From commons.wikimedia.org
Original file (SVG file, nominally 334 × 254 pixels, file size 42 KB) Iron Electron Configuration Full The shorthand electron configuration (or noble gas configuration) as well as. Its electron configuration is [ar] 3d 6 4s 2, which means it has two valence. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. The chemical element iron has the atomic number 26 and the symbol fe (from latin:. Iron Electron Configuration Full.
From www.haikudeck.com
Iron by Claudia Stevens Iron Electron Configuration Full Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Iron is a transition metal with symbol fe and atomic number 26. Its electron configuration is [ar] 3d 6 4s 2, which means it has two valence. It’s a transition metal from group 8 of the periodic table’s first transition series. Since 1s. Iron Electron Configuration Full.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Iron Electron Configuration Full Since 1s can only hold two electrons the next 2. In writing the electron configuration for iron the first two electrons will go in the 1s orbital. Electron configuration chart of all elements is mentioned in the table below. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Iron is a transition. Iron Electron Configuration Full.
From www.youtube.com
Iron (Fe) Electron Configuration YouTube Iron Electron Configuration Full Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Iron is a transition metal with symbol fe and atomic number 26. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Since 1s can only hold two electrons the next 2. Its electron configuration is [ar] 3d. Iron Electron Configuration Full.
From www.sciencephoto.com
Iron, atomic structure Stock Image C013/1539 Science Photo Library Iron Electron Configuration Full Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. Iron is a transition metal with symbol fe and atomic number 26. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. It’s a transition metal from group 8 of the periodic table’s first. Iron Electron Configuration Full.
From www.bigstockphoto.com
Iron. Atom Structure Vector & Photo Bigstock Iron Electron Configuration Full It’s a transition metal from group 8 of the periodic table’s first transition series. Electron configuration chart of all elements is mentioned in the table below. Since 1s can only hold two electrons the next 2. Its electron configuration is [ar] 3d 6 4s 2, which means it has two valence. The shorthand electron configuration (or noble gas configuration) as. Iron Electron Configuration Full.
From material-properties.org
Iron Protons Neutrons Electrons Electron Configuration Iron Electron Configuration Full In writing the electron configuration for iron the first two electrons will go in the 1s orbital. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. Electron configuration chart of all elements is mentioned in. Iron Electron Configuration Full.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Iron Electron Configuration Full The chemical element iron has the atomic number 26 and the symbol fe (from latin: Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. Iron is a transition metal with symbol fe and atomic number 26. Find the full electronic configuration and valence electrons of any periodic element using this. Iron Electron Configuration Full.
From justanothershop-a-holic.blogspot.com
Electron Configuration Of Iron Full worksheet today Iron Electron Configuration Full Since 1s can only hold two electrons the next 2. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The chemical element iron has the atomic number 26 and the symbol fe (from latin: In writing the electron configuration for iron the first two electrons will go in the 1s orbital. Its. Iron Electron Configuration Full.
From sciencenotes.org
Iron Atom Science Notes and Projects Iron Electron Configuration Full Iron is a transition metal with symbol fe and atomic number 26. It’s a transition metal from group 8 of the periodic table’s first transition series. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic. Iron Electron Configuration Full.
From www.webelements.com
Elements Periodic Table » Iron » properties of free atoms Iron Electron Configuration Full Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. It’s a transition metal from group 8 of the periodic table’s first transition series. Since 1s can only hold two electrons the next 2. Electron configuration chart of all elements is mentioned in the table below. Its electron configuration is [ar] 3d 6. Iron Electron Configuration Full.
From www.slideserve.com
PPT More on Electron Configurations PowerPoint Presentation, free Iron Electron Configuration Full Its electron configuration is [ar] 3d 6 4s 2, which means it has two valence. Electron configuration chart of all elements is mentioned in the table below. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. Iron is on the fourth row of. Iron Electron Configuration Full.
From stock.adobe.com
Iron atomic structure has atomic number, atomic mass, electron Iron Electron Configuration Full The chemical element iron has the atomic number 26 and the symbol fe (from latin: Electron configuration chart of all elements is mentioned in the table below. Its electron configuration is [ar] 3d 6 4s 2, which means it has two valence. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Iron. Iron Electron Configuration Full.
From www.chemistry-online.com
Iron Chemistry Online Iron Electron Configuration Full Iron is a transition metal with symbol fe and atomic number 26. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. The shorthand electron configuration (or noble gas configuration) as well as. It’s a transition metal from group 8 of the periodic table’s first transition series. Its electron configuration is. Iron Electron Configuration Full.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Iron Electron Configuration Full Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. Iron is a transition metal with symbol fe and atomic number 26. Since 1s can only hold two electrons the next 2. Electron configuration chart of all elements is mentioned in the table below. The chemical element iron has the atomic number 26 and the symbol fe (from latin:. Iron Electron Configuration Full.
From www.britannica.com
Iron Element, Occurrence, Uses, Properties, & Compounds Britannica Iron Electron Configuration Full The shorthand electron configuration (or noble gas configuration) as well as. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Electron configuration chart of all elements is mentioned in the table below. Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. Iron. Iron Electron Configuration Full.
From www.shutterstock.com
54 imágenes, fotos de stock, objetos en 3D y vectores sobre Fe electron Iron Electron Configuration Full Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. In writing the electron configuration for iron the first. Iron Electron Configuration Full.
From www.alamy.com
Iron (Fe). Diagram of the nuclear composition, electron configuration Iron Electron Configuration Full Electron configuration chart of all elements is mentioned in the table below. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. It’s a transition metal from group 8 of the periodic table’s first transition series. Iron is a transition metal with. Iron Electron Configuration Full.
From justanothershop-a-holic.blogspot.com
Electron Configuration Of Iron Full worksheet today Iron Electron Configuration Full The shorthand electron configuration (or noble gas configuration) as well as. Since 1s can only hold two electrons the next 2. In writing the electron configuration for iron the first two electrons will go in the 1s orbital. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. It’s a transition metal from. Iron Electron Configuration Full.
From www.youtube.com
Iron electronic configuration How to Write Iron electronic Iron Electron Configuration Full Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. The shorthand electron configuration (or noble gas configuration) as well as. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Its electron configuration is [ar] 3d 6 4s 2, which means it has. Iron Electron Configuration Full.
From www.alamy.com
Diagram of the nuclear composition, electron configuration, and valence Iron Electron Configuration Full Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number #26#. Since 1s can only hold two electrons the next 2. The shorthand electron configuration (or noble gas configuration) as well as. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Electron configuration chart. Iron Electron Configuration Full.
From www.alamy.com
Symbol and electron diagram for Iron illustration Stock Vector Image Iron Electron Configuration Full Its electron configuration is [ar] 3d 6 4s 2, which means it has two valence. Since 1s can only hold two electrons the next 2. Electron configuration chart of all elements is mentioned in the table below. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. The shorthand electron configuration (or noble gas configuration) as well as. Iron. Iron Electron Configuration Full.
From pnghut.com
Electron Configuration Atomic Orbital Shell Energy Level Iron Iron Electron Configuration Full Its electron configuration is [ar] 3d 6 4s 2, which means it has two valence. Since 1s can only hold two electrons the next 2. The shorthand electron configuration (or noble gas configuration) as well as. Iron is a transition metal with symbol fe and atomic number 26. The chemical element iron has the atomic number 26 and the symbol. Iron Electron Configuration Full.
From guidedehartfederalist.z21.web.core.windows.net
Iron Electron Dot Diagram Iron Electron Configuration Full In writing the electron configuration for iron the first two electrons will go in the 1s orbital. Electron configuration chart of all elements is mentioned in the table below. It’s a transition metal from group 8 of the periodic table’s first transition series. Since 1s can only hold two electrons the next 2. Find the full electronic configuration and valence. Iron Electron Configuration Full.
From mavink.com
Electron Orbital Configuration Chart Iron Electron Configuration Full It’s a transition metal from group 8 of the periodic table’s first transition series. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and. Since 1s can only hold two electrons the next 2. In writing the electron configuration for iron the first two electrons will go in the 1s orbital. The shorthand electron configuration (or noble gas configuration). Iron Electron Configuration Full.