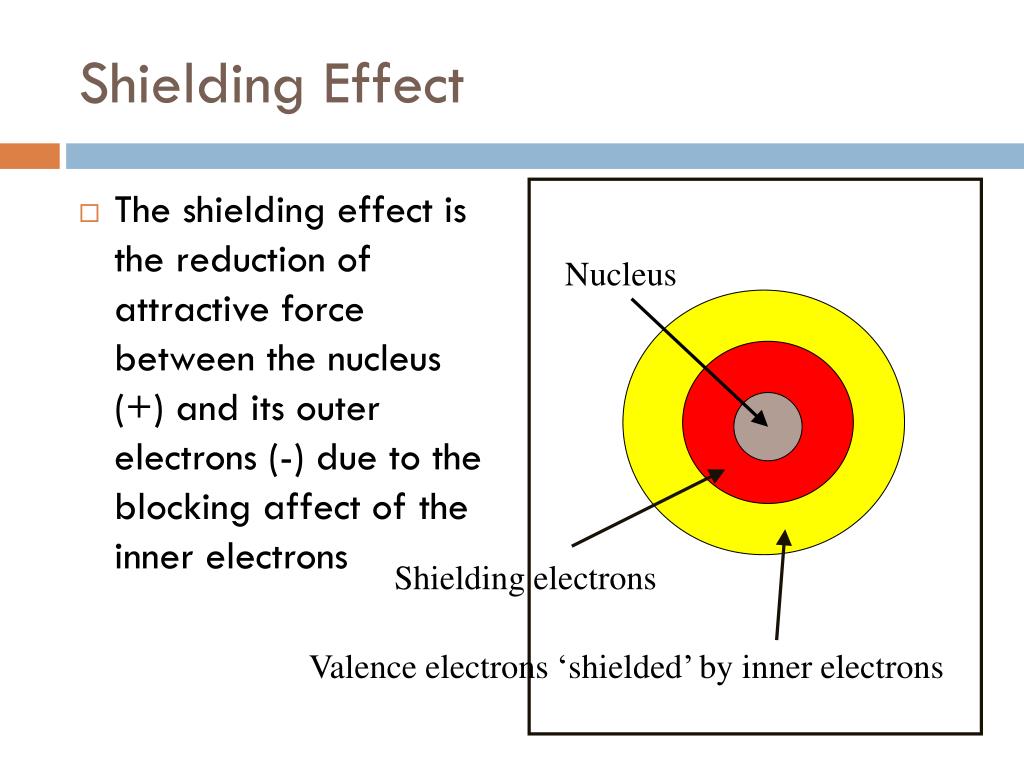
What Is Shielding In Chemistry . We can predict basic properties of elements by using shielding and penetration characteristics to assess basic trends. The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of the shielding effect. The basic principle of nmr is to apply an external magnetic field called b0 and measure the frequency at which the nucleus. Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. Penetration and shielding are two underlying principles in determining the physical and chemical properties of elements.
from mungfali.com
Penetration and shielding are two underlying principles in determining the physical and chemical properties of elements. The basic principle of nmr is to apply an external magnetic field called b0 and measure the frequency at which the nucleus. We can predict basic properties of elements by using shielding and penetration characteristics to assess basic trends. The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of the shielding effect. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge.
What Is Shielding Effect
What Is Shielding In Chemistry The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of the shielding effect. The basic principle of nmr is to apply an external magnetic field called b0 and measure the frequency at which the nucleus. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of the shielding effect. We can predict basic properties of elements by using shielding and penetration characteristics to assess basic trends. Penetration and shielding are two underlying principles in determining the physical and chemical properties of elements. Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge.
From socratic.org
How are shielding effect and atomic radius related? Socratic What Is Shielding In Chemistry Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The effective nuclear charge experienced by an. What Is Shielding In Chemistry.
From www.slideserve.com
PPT Chapter 8 Periodic Properties of the Elements PowerPoint What Is Shielding In Chemistry Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge. Penetration and shielding are two underlying principles in determining the physical and chemical properties of elements. We can predict basic properties of elements by using shielding and penetration characteristics to assess basic trends.. What Is Shielding In Chemistry.
From www.slideserve.com
PPT Chapter 6 The Periodic Table PowerPoint Presentation, free What Is Shielding In Chemistry The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of the shielding effect. The basic principle of nmr is to apply an external magnetic field called b0 and measure the frequency at which the nucleus. Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends. What Is Shielding In Chemistry.
From www.researchgate.net
Aromatic ringinduced shielding and deshielding zones. Download What Is Shielding In Chemistry Penetration and shielding are two underlying principles in determining the physical and chemical properties of elements. The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of the shielding effect. Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and. What Is Shielding In Chemistry.
From www.chegg.com
Consider The Process Of Shielding In Atoms, Using What Is Shielding In Chemistry Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. We can predict basic properties of elements by using shielding and penetration characteristics to assess basic trends. Find out the factors that determine the magnitude of shielding effect and how it explains the periodic. What Is Shielding In Chemistry.
From www.breakingatom.com
Shielding What Is Shielding In Chemistry Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. We can predict basic properties of elements by using shielding and penetration characteristics to assess basic trends. Find out the factors that determine the magnitude of shielding effect and how it explains the periodic. What Is Shielding In Chemistry.
From wisc.pb.unizin.org
Core and Valence Electrons, Shielding, Zeff (M7Q8) UWMadison What Is Shielding In Chemistry The basic principle of nmr is to apply an external magnetic field called b0 and measure the frequency at which the nucleus. Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge. Electrons in an \ (s\) orbital can shield \ (p\) electrons. What Is Shielding In Chemistry.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity What Is Shielding In Chemistry The basic principle of nmr is to apply an external magnetic field called b0 and measure the frequency at which the nucleus. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. Penetration and shielding are two underlying principles in determining the physical and. What Is Shielding In Chemistry.
From www.researchgate.net
a) Orientation of the chemical shielding tensor in ethylene, b) results What Is Shielding In Chemistry Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge. Penetration and shielding are two underlying principles in determining the physical and chemical properties of elements. We can predict basic properties of elements by using shielding and penetration characteristics to assess basic trends.. What Is Shielding In Chemistry.
From www.youtube.com
Orbital shielding and YouTube What Is Shielding In Chemistry Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge. We can predict basic properties of elements by using shielding and penetration characteristics to assess basic trends. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level. What Is Shielding In Chemistry.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting What Is Shielding In Chemistry Penetration and shielding are two underlying principles in determining the physical and chemical properties of elements. The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of the shielding effect. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \. What Is Shielding In Chemistry.
From www.youtube.com
Chapter 3 Shielding Effect and Atomic Size YouTube What Is Shielding In Chemistry Penetration and shielding are two underlying principles in determining the physical and chemical properties of elements. Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge. The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of. What Is Shielding In Chemistry.
From www.showme.com
Electron shielding Science, Chemistry, Electrons, electron shielding What Is Shielding In Chemistry We can predict basic properties of elements by using shielding and penetration characteristics to assess basic trends. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of. What Is Shielding In Chemistry.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID4499242 What Is Shielding In Chemistry The basic principle of nmr is to apply an external magnetic field called b0 and measure the frequency at which the nucleus. The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of the shielding effect. Penetration and shielding are two underlying principles in determining the physical and chemical properties of elements. Electrons in. What Is Shielding In Chemistry.
From chem.libretexts.org
3.2 Shielding Chemistry LibreTexts What Is Shielding In Chemistry Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge. We can predict basic properties of elements by using shielding and penetration characteristics to assess basic trends. Penetration and shielding are two underlying principles in determining the physical and chemical properties of elements.. What Is Shielding In Chemistry.
From mungfali.com
What Is Shielding Effect What Is Shielding In Chemistry Penetration and shielding are two underlying principles in determining the physical and chemical properties of elements. Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because. What Is Shielding In Chemistry.
From www.youtube.com
Shielding and Deshielding H NMR Spectroscopy YouTube What Is Shielding In Chemistry The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of the shielding effect. The basic principle of nmr is to apply an external magnetic field called b0 and measure the frequency at which the nucleus. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of. What Is Shielding In Chemistry.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID4499242 What Is Shielding In Chemistry The basic principle of nmr is to apply an external magnetic field called b0 and measure the frequency at which the nucleus. Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge. We can predict basic properties of elements by using shielding and. What Is Shielding In Chemistry.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube What Is Shielding In Chemistry The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of the shielding effect. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The basic principle of nmr is to apply an external magnetic field called b0. What Is Shielding In Chemistry.
From www.youtube.com
Shielding Effect YouTube What Is Shielding In Chemistry The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of the shielding effect. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The basic principle of nmr is to apply an external magnetic field called b0. What Is Shielding In Chemistry.
From www.youtube.com
Shielding Effect and Ionization Energy Class9 Chemistry YouTube What Is Shielding In Chemistry We can predict basic properties of elements by using shielding and penetration characteristics to assess basic trends. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of. What Is Shielding In Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Shielded What Is Shielding In Chemistry Penetration and shielding are two underlying principles in determining the physical and chemical properties of elements. We can predict basic properties of elements by using shielding and penetration characteristics to assess basic trends. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. Find. What Is Shielding In Chemistry.
From www.showme.com
AP 2.11a ENC and Electron Shielding Science, Atoms, Periodic Table What Is Shielding In Chemistry Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of the shielding effect. The basic principle of nmr is to apply an external magnetic field called b0. What Is Shielding In Chemistry.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Shielding In Chemistry The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of the shielding effect. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. Penetration and shielding are two underlying principles in determining the physical and chemical properties. What Is Shielding In Chemistry.
From www.showme.com
Shielding constant Science, Atoms, Elements, Chemistry ShowMe What Is Shielding In Chemistry Penetration and shielding are two underlying principles in determining the physical and chemical properties of elements. Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because. What Is Shielding In Chemistry.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Shielding In Chemistry Penetration and shielding are two underlying principles in determining the physical and chemical properties of elements. We can predict basic properties of elements by using shielding and penetration characteristics to assess basic trends. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The. What Is Shielding In Chemistry.
From byjus.com
What is shielding and deshielding in NMR? Give an example? What Is Shielding In Chemistry Penetration and shielding are two underlying principles in determining the physical and chemical properties of elements. Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge. We can predict basic properties of elements by using shielding and penetration characteristics to assess basic trends.. What Is Shielding In Chemistry.
From www.breakingatom.com
Shielding What Is Shielding In Chemistry We can predict basic properties of elements by using shielding and penetration characteristics to assess basic trends. Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge. Penetration and shielding are two underlying principles in determining the physical and chemical properties of elements.. What Is Shielding In Chemistry.
From www.nuclear-power.com
Shielding of Alpha Radiation Types & Uses What Is Shielding In Chemistry Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge. The basic principle of nmr is to apply an external magnetic field called b0 and measure the frequency at which the nucleus. The effective nuclear charge experienced by an electron is less than. What Is Shielding In Chemistry.
From www.youtube.com
Shielding Effect in the Periodic Table Chemistry YouTube What Is Shielding In Chemistry Penetration and shielding are two underlying principles in determining the physical and chemical properties of elements. The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of the shielding effect. The basic principle of nmr is to apply an external magnetic field called b0 and measure the frequency at which the nucleus. Find out. What Is Shielding In Chemistry.
From www.youtube.com
Shielding Deshielding Effects Explained Nucleus Get better grade in What Is Shielding In Chemistry Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge. The effective nuclear charge experienced by an electron is less than the actual nuclear charge because of the shielding effect. We can predict basic properties of elements by using shielding and penetration characteristics. What Is Shielding In Chemistry.
From www.youtube.com
Shielding Effect Lecture 07 Chapter 03 Chemistry Class 9th YouTube What Is Shielding In Chemistry Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. Penetration and shielding are two underlying principles. What Is Shielding In Chemistry.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting What Is Shielding In Chemistry Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The basic principle of nmr is to apply an external magnetic field called b0 and measure the frequency at which the nucleus. Find out the factors that determine the magnitude of shielding effect and. What Is Shielding In Chemistry.
From www.slideserve.com
PPT Chapter 6 Chemical Bonding PowerPoint Presentation, free What Is Shielding In Chemistry We can predict basic properties of elements by using shielding and penetration characteristics to assess basic trends. Find out the factors that determine the magnitude of shielding effect and how it explains the periodic trends of atomic radius, ionization energy, and effective nuclear charge. The basic principle of nmr is to apply an external magnetic field called b0 and measure. What Is Shielding In Chemistry.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free What Is Shielding In Chemistry The basic principle of nmr is to apply an external magnetic field called b0 and measure the frequency at which the nucleus. Electrons in an \ (s\) orbital can shield \ (p\) electrons at the same energy level because of the spherical shape of the \ (s\) orbital. The effective nuclear charge experienced by an electron is less than the. What Is Shielding In Chemistry.