Does Potassium Sulfide And Zinc Sulfate Precipitate . A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Whether or not such a reaction. The following chart shows the solubility of various ionic compounds in water at 1 atm pressure and room temperature (approx. This guide will show how to. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Updated on october 09, 2019. The two possible products for this combination are kno. Potassium sulfate and barium nitrate; Lithium chloride and silver acetate) solution. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Most elements are rarely found in their pure form.
from www.numerade.com
Lithium chloride and silver acetate) solution. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Whether or not such a reaction. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Most elements are rarely found in their pure form. Potassium sulfate and barium nitrate; The following chart shows the solubility of various ionic compounds in water at 1 atm pressure and room temperature (approx. Updated on october 09, 2019. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility.
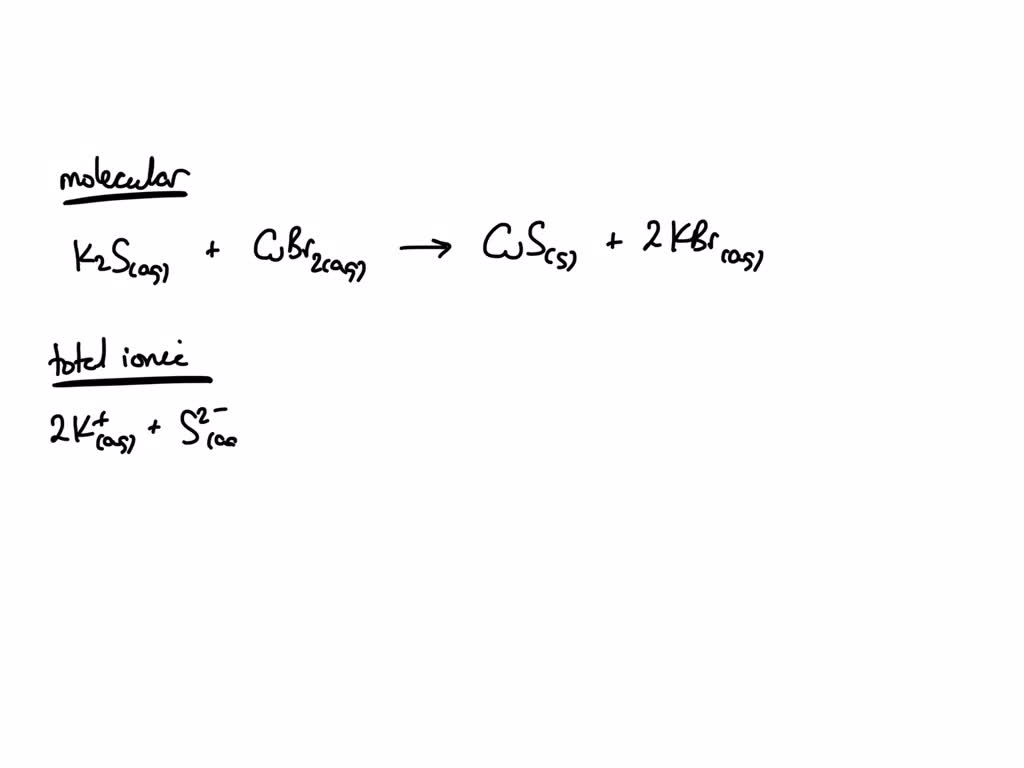
SOLVED Write the net ionic equation for the precipitation reaction
Does Potassium Sulfide And Zinc Sulfate Precipitate Updated on october 09, 2019. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Whether or not such a reaction. The two possible products for this combination are kno. Lithium chloride and silver acetate) solution. Most elements are rarely found in their pure form. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Potassium sulfate and barium nitrate; Updated on october 09, 2019. The following chart shows the solubility of various ionic compounds in water at 1 atm pressure and room temperature (approx. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. This guide will show how to.
From www.slideshare.net
Precipitates Does Potassium Sulfide And Zinc Sulfate Precipitate This guide will show how to. Most elements are rarely found in their pure form. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Whether or not such a reaction. Potassium sulfate and barium nitrate; Updated on october 09, 2019. A substance will precipitate when solution conditions are such that. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED Write the net ionic equation for the precipitation reaction Does Potassium Sulfide And Zinc Sulfate Precipitate Most elements are rarely found in their pure form. Lithium chloride and silver acetate) solution. Potassium sulfate and barium nitrate; The two possible products for this combination are kno. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. The following chart shows the solubility of various ionic compounds in water at 1 atm pressure. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED Does precipitate form when A and B are mixed? empirical formula Does Potassium Sulfide And Zinc Sulfate Precipitate When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. The two possible products for this combination are kno. Potassium sulfate and barium nitrate; Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Updated on october 09, 2019. Most elements are. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.youtube.com
Does sodium sulfide (Na2S) and zinc nitrate (Zn(NO3)2 precipitate Does Potassium Sulfide And Zinc Sulfate Precipitate A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. The following chart shows the solubility of various ionic compounds in water at 1 atm pressure and room temperature (approx. The. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.chegg.com
Solved Complete the table below by deciding whether a Does Potassium Sulfide And Zinc Sulfate Precipitate The following chart shows the solubility of various ionic compounds in water at 1 atm pressure and room temperature (approx. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Potassium sulfate and barium nitrate; Lithium chloride and silver acetate) solution. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED solution A solution B Does a precipitate form when A and B are Does Potassium Sulfide And Zinc Sulfate Precipitate This guide will show how to. Most elements are rarely found in their pure form. The following chart shows the solubility of various ionic compounds in water at 1 atm pressure and room temperature (approx. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Updated on october 09, 2019. Whether or. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Does Potassium Sulfide And Zinc Sulfate Precipitate A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Updated on october 09, 2019. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Potassium. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.coursehero.com
[Solved] Give the formulas of the following compounds potassium Does Potassium Sulfide And Zinc Sulfate Precipitate This guide will show how to. Potassium sulfate and barium nitrate; A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Most elements are rarely found in their pure form. The following chart shows the solubility of various ionic compounds in water at 1 atm pressure and room temperature (approx. When. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Does Potassium Sulfide And Zinc Sulfate Precipitate Updated on october 09, 2019. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. The following chart shows the solubility of various ionic compounds in water at 1 atm pressure and room temperature (approx. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Potassium sulfate. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.chegg.com
Solved Complete The Table Below By Deciding Whether A Pre... Does Potassium Sulfide And Zinc Sulfate Precipitate Whether or not such a reaction. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Most elements are rarely found in their pure form. Potassium sulfate and barium nitrate; Updated on october 09, 2019. A typical. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From projectopenletter.com
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form Does Potassium Sulfide And Zinc Sulfate Precipitate A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. This guide will show how to. Whether or not such a reaction. Potassium sulfate and barium nitrate; A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Lithium chloride and silver acetate) solution. Most elements are. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED 'Does a empirical formula of precipitate precipitate form when Does Potassium Sulfide And Zinc Sulfate Precipitate Updated on october 09, 2019. Whether or not such a reaction. Potassium sulfate and barium nitrate; A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. This guide will show how to. The two possible products for this combination are kno. The following chart shows the solubility of various ionic compounds in water at 1. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From projectopenletter.com
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form Does Potassium Sulfide And Zinc Sulfate Precipitate When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. The two possible products for this combination are kno. Potassium sulfate and barium nitrate; Whether or not such a reaction. This guide will show how to. Most elements are rarely found in their pure form. The following chart shows the solubility of. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.chegg.com
Solved 4. The fo solutions of zinc sulfate and potassium Does Potassium Sulfide And Zinc Sulfate Precipitate This guide will show how to. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Most elements are rarely found in their pure form. Whether or not such a reaction. The two possible products for this combination are kno. When two aqueous solutions of ionic compounds are mixed together,. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED Does a empirical formula of precipitate precipitate form when A Does Potassium Sulfide And Zinc Sulfate Precipitate Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Potassium sulfate and barium nitrate; The following chart shows the solubility of various ionic compounds in water at 1 atm pressure and room temperature (approx. Updated on october 09, 2019. Whether or not such a reaction. A substance will precipitate. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.youtube.com
How to Write the Net Ionic Equation for K2S + ZnSO4 = K2SO4 + ZnS YouTube Does Potassium Sulfide And Zinc Sulfate Precipitate A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. The two possible products for this combination are kno. Updated on october 09, 2019. A typical precipitation reaction occurs when an aqueous solution of barium. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED Complete the table below by deciding whether precipitate forms Does Potassium Sulfide And Zinc Sulfate Precipitate Whether or not such a reaction. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Potassium sulfate and barium nitrate; Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Most elements are rarely found in their pure form. Updated. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.researchgate.net
Zeta potential of zinc sulfide precipitates (circles) and mineral Does Potassium Sulfide And Zinc Sulfate Precipitate Updated on october 09, 2019. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. The two possible products for this combination are kno. Most elements are rarely found in their pure form. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Whether or not. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.chegg.com
Solved Complete the table below by deciding whether a Does Potassium Sulfide And Zinc Sulfate Precipitate Updated on october 09, 2019. Lithium chloride and silver acetate) solution. This guide will show how to. Potassium sulfate and barium nitrate; Most elements are rarely found in their pure form. The two possible products for this combination are kno. Whether or not such a reaction. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.fishersci.ca
Potassium sulfite, 90, pure, Thermo Scientific Chemicals Fisher Does Potassium Sulfide And Zinc Sulfate Precipitate Potassium sulfate and barium nitrate; Updated on october 09, 2019. Most elements are rarely found in their pure form. The following chart shows the solubility of various ionic compounds in water at 1 atm pressure and room temperature (approx. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. This guide will. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.youtube.com
Does Potassium hydroxide (KOH) and Zinc sulfate (ZnSO4) form a Does Potassium Sulfide And Zinc Sulfate Precipitate Most elements are rarely found in their pure form. Updated on october 09, 2019. The two possible products for this combination are kno. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. The following chart shows the solubility of various ionic compounds in water at 1 atm pressure and room. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From projectopenletter.com
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form Does Potassium Sulfide And Zinc Sulfate Precipitate Updated on october 09, 2019. Lithium chloride and silver acetate) solution. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Whether or not such a reaction. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. The two possible products for. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.chegg.com
Solved O CHEMICAL REACTIONS Predicting precipitation Does Potassium Sulfide And Zinc Sulfate Precipitate Lithium chloride and silver acetate) solution. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Updated on october 09, 2019. Whether or not such a reaction. Most elements are rarely found in their pure form. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.chegg.com
Solved does zinc bromide and sodium sulfide precipitate and Does Potassium Sulfide And Zinc Sulfate Precipitate The two possible products for this combination are kno. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Whether or not such a reaction. Lithium chloride and silver acetate) solution. This guide will show how to. The following chart shows the solubility of various ionic compounds in water at 1. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.chegg.com
Solved O CHEMICAL REACTIONS Predicting precipitation Does Potassium Sulfide And Zinc Sulfate Precipitate Most elements are rarely found in their pure form. Potassium sulfate and barium nitrate; When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Lithium chloride and silver acetate) solution. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. Precipitation reactions occur. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED Complete the table below by deciding whether precipitate forms Does Potassium Sulfide And Zinc Sulfate Precipitate Most elements are rarely found in their pure form. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Updated on october 09, 2019. The following chart shows the solubility of various ionic compounds in water at 1 atm pressure and room temperature (approx. Lithium chloride and silver acetate) solution. Whether or. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From questions.kunduz.com
Complete the table below by deciding whe... Chemistry Does Potassium Sulfide And Zinc Sulfate Precipitate A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Lithium chloride and silver acetate) solution. Potassium. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.youtube.com
Does zinc sulfate (ZnSO4) and potassium sulfide (K2S) form a Does Potassium Sulfide And Zinc Sulfate Precipitate This guide will show how to. A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate.. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.slideserve.com
PPT Anion tests PowerPoint Presentation, free download ID3821627 Does Potassium Sulfide And Zinc Sulfate Precipitate When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Lithium chloride and silver acetate) solution. This guide will show how to. The following chart shows the solubility of various ionic compounds in water at 1 atm pressure and room temperature (approx. The two possible products for this combination are kno. A. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.slideshare.net
Precipitates Does Potassium Sulfide And Zinc Sulfate Precipitate A typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. The two possible products for this combination are kno. Whether or not such a reaction. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. This guide will show how to. Lithium chloride. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.chegg.com
Solved solution A solution B Does a precipitate form when A Does Potassium Sulfide And Zinc Sulfate Precipitate This guide will show how to. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Lithium chloride and silver acetate) solution. The two possible products for this combination are kno. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Whether or not such. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.pw.live
Potassium Sulfite Formula, Structure, Properties, Uses Does Potassium Sulfide And Zinc Sulfate Precipitate Most elements are rarely found in their pure form. The following chart shows the solubility of various ionic compounds in water at 1 atm pressure and room temperature (approx. Potassium sulfate and barium nitrate; Lithium chloride and silver acetate) solution. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Whether or. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.numerade.com
SOLVED Does the empirical formula of a precipitate form when A and B Does Potassium Sulfide And Zinc Sulfate Precipitate When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Lithium chloride and silver acetate) solution. Potassium sulfate and barium nitrate; Whether or not such a reaction. The following chart shows the solubility of various ionic compounds. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.aquaportail.com
Sulfate définition illustrée et explications Does Potassium Sulfide And Zinc Sulfate Precipitate Whether or not such a reaction. Updated on october 09, 2019. Most elements are rarely found in their pure form. The following chart shows the solubility of various ionic compounds in water at 1 atm pressure and room temperature (approx. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Potassium sulfate and barium nitrate;. Does Potassium Sulfide And Zinc Sulfate Precipitate.
From www.sciencephoto.com
Zinc sulphide precipitate, 1 of 3 Stock Image C036/3904 Science Does Potassium Sulfide And Zinc Sulfate Precipitate A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Lithium chloride and silver acetate) solution. Whether or not such a reaction. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. The two possible products for this combination are kno. Most elements are rarely found in. Does Potassium Sulfide And Zinc Sulfate Precipitate.