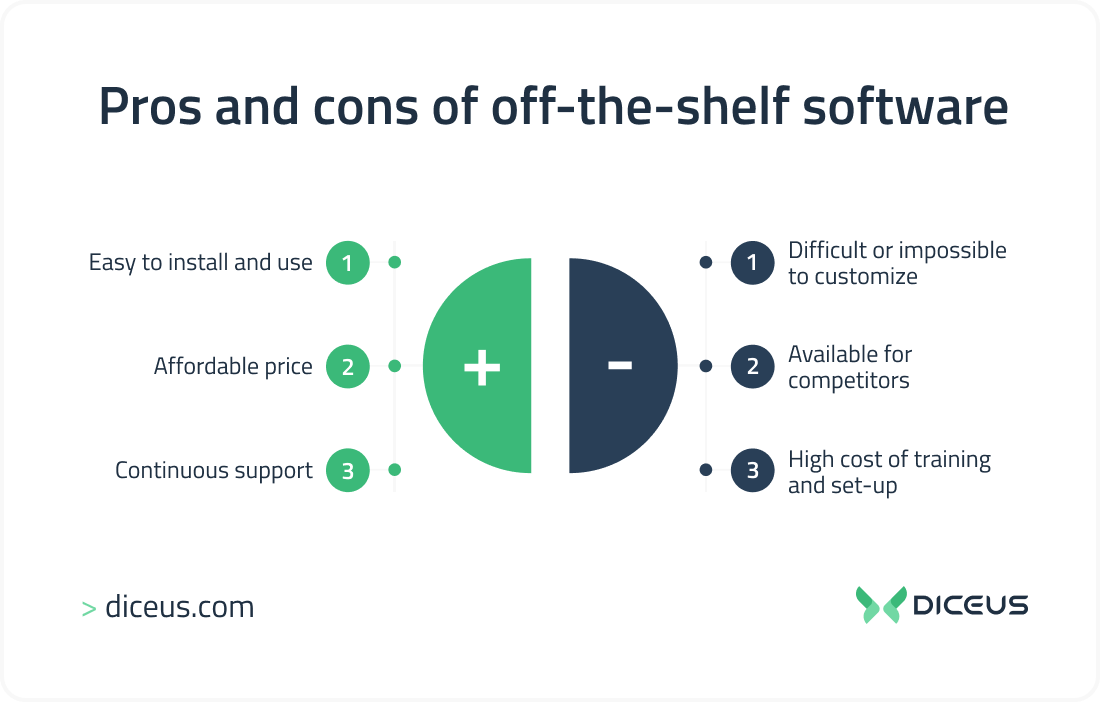
Off The Shelf Software Application . We look at the pros & cons of each. The fda defines ots software as. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations.
from diceus.com
A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. The fda defines ots software as. We look at the pros & cons of each.
Custom vs OfftheShelf Software Advantages & Disadvantages
Off The Shelf Software Application Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. The fda defines ots software as. We look at the pros & cons of each. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot.
From www.thecyberiatech.com
Off Shelf Software in 2023 Benefits and Drawbacks Explained Off The Shelf Software Application We look at the pros & cons of each. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. The fda defines ots software as. Off The Shelf Software Application.
From itechnolabs.ca
What is a COTS Software [Updated 2024] Off The Shelf Software Application A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. We look at the pros & cons of each. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. The fda defines ots software as. Off The Shelf Software Application.
From www.whitecapcanada.com
Decide Between OffTheShelf And Custom Software Whitecap Canada Off The Shelf Software Application We look at the pros & cons of each. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. The fda defines ots software as. Off The Shelf Software Application.
From itechnolabs.ca
What is a COTS Software [Updated 2024] Off The Shelf Software Application Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. We look at the pros & cons of each. The fda defines ots software as. Off The Shelf Software Application.
From www.rezaid.co.uk
What is Off the Shelf Software? Off The Shelf Software Application A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. We look at the pros & cons of each. The fda defines ots software as. Off The Shelf Software Application.
From blog.foreworth.com
The Lowdown on OfftheShelf Software Advantages and Disadvantages Off The Shelf Software Application We look at the pros & cons of each. The fda defines ots software as. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. Off The Shelf Software Application.
From www.koder.ly
Offtheshelf Software vs Bespoke Software Off The Shelf Software Application The fda defines ots software as. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. We look at the pros & cons of each. Off The Shelf Software Application.
From stratoflow.com
Off the Shelf vs Custom Software Pros & Cons + Examples Stratoflow Off The Shelf Software Application The fda defines ots software as. We look at the pros & cons of each. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off The Shelf Software Application.
From www.appventurez.com
Custom Software vs OfftheShelf Software Off The Shelf Software Application Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. We look at the pros & cons of each. The fda defines ots software as. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off The Shelf Software Application.
From xbsoftware.com
Custom Software vs OfftheShelf Software XB Software Off The Shelf Software Application The fda defines ots software as. We look at the pros & cons of each. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. Off The Shelf Software Application.
From www.codecreatorsinc.com
Custom Software vs OfftheShelf Software Code Creators Inc Off The Shelf Software Application A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. We look at the pros & cons of each. The fda defines ots software as. Off The Shelf Software Application.
From dcastalia.com
Custom Software for A Better Choice Over Offshelf Software Off The Shelf Software Application Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. We look at the pros & cons of each. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. The fda defines ots software as. Off The Shelf Software Application.
From sparkbusinessworks.com
Building Custom Software vs OfftheShelf Advantages and Disadvantages Off The Shelf Software Application A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. The fda defines ots software as. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. We look at the pros & cons of each. Off The Shelf Software Application.
From stratoflow.com
Off the Shelf vs Custom Software Pros & Cons + Examples Stratoflow Off The Shelf Software Application A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. We look at the pros & cons of each. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. The fda defines ots software as. Off The Shelf Software Application.
From itechnolabs.ca
What is a COTS Software [Updated 2024] Off The Shelf Software Application A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. The fda defines ots software as. We look at the pros & cons of each. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. Off The Shelf Software Application.
From onextdigital.com
Unveiling Off the Shelf Software The Digital Revolution Simplified Off The Shelf Software Application A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. The fda defines ots software as. We look at the pros & cons of each. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. Off The Shelf Software Application.
From moreapp.com
OfftheShelf Software vs Custom Software MoreApp Blog Off The Shelf Software Application Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. The fda defines ots software as. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. We look at the pros & cons of each. Off The Shelf Software Application.
From andersenlab.com
Custom Software vs OfftheShelf Pros & Cons Off The Shelf Software Application Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. We look at the pros & cons of each. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. The fda defines ots software as. Off The Shelf Software Application.
From innolitics.com
OffTheShelf Software Best Practices, FAQs, and Examples Off The Shelf Software Application Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. The fda defines ots software as. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. We look at the pros & cons of each. Off The Shelf Software Application.
From www.weetechsolution.com
10 Best OfftheShelf Software Examples Ready to Transform Your Business Off The Shelf Software Application Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. We look at the pros & cons of each. The fda defines ots software as. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off The Shelf Software Application.
From www.valuecoders.com
Custom Software vs. OfftheShelf Startup Choices Off The Shelf Software Application A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. The fda defines ots software as. We look at the pros & cons of each. Off The Shelf Software Application.
From www.atlascode.com
Offtheshelf Software Advantages and Disadvantages [2020] Off The Shelf Software Application A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. The fda defines ots software as. We look at the pros & cons of each. Off The Shelf Software Application.
From longwallshelf.blogspot.com
Commercial Off The Shelf Software Long Wall Shelf Off The Shelf Software Application The fda defines ots software as. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. We look at the pros & cons of each. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. Off The Shelf Software Application.
From onextdigital.com
Unveiling Off the Shelf Software The Digital Revolution Simplified Off The Shelf Software Application We look at the pros & cons of each. The fda defines ots software as. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off The Shelf Software Application.
From www.botreetechnologies.com
Customized Software What is it, Types, and Examples Off The Shelf Software Application A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. We look at the pros & cons of each. The fda defines ots software as. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. Off The Shelf Software Application.
From www.weetechsolution.com
10 Best OfftheShelf Software Examples Ready to Transform Your Business Off The Shelf Software Application The fda defines ots software as. We look at the pros & cons of each. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. Off The Shelf Software Application.
From itenterprise.co.uk
The Complete Advantages and Disadvantages of Off the Shelf Software Off The Shelf Software Application Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. We look at the pros & cons of each. The fda defines ots software as. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off The Shelf Software Application.
From www.web-alliance.co.uk
Pros and cons of offtheshelf software. Off The Shelf Software Application We look at the pros & cons of each. The fda defines ots software as. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. Off The Shelf Software Application.
From simpat.tech
5 OffTheShelf Software Advantages and Disadvantages Simpat Tech Off The Shelf Software Application A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. We look at the pros & cons of each. The fda defines ots software as. Off The Shelf Software Application.
From decode.agency
Choosing between custom vs. offtheshelf software Off The Shelf Software Application We look at the pros & cons of each. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. The fda defines ots software as. Off The Shelf Software Application.
From morioh.com
Custom Software vs Offtheshelf Software How to select a better one Off The Shelf Software Application The fda defines ots software as. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. We look at the pros & cons of each. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. Off The Shelf Software Application.
From radixweb.com
Difference between Bespoke Application Development and OfftheShelf Off The Shelf Software Application The fda defines ots software as. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. We look at the pros & cons of each. Off The Shelf Software Application.
From diceus.com
Custom vs OfftheShelf Software Advantages & Disadvantages Off The Shelf Software Application Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. We look at the pros & cons of each. The fda defines ots software as. Off The Shelf Software Application.
From dignitas.digital
Using off the shelf solution or Custom Software Dignitas Digital Off The Shelf Software Application Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. The fda defines ots software as. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. We look at the pros & cons of each. Off The Shelf Software Application.
From www.weetechsolution.com
10 Best OfftheShelf Software Examples Ready to Transform Your Business Off The Shelf Software Application The fda defines ots software as. We look at the pros & cons of each. A generally available software component, used by a medical device manufacturer for which the manufacturer cannot. Off the shelf software are software applications designed for a broad range of customers, offering a comprehensive set of features to streamline operations. Off The Shelf Software Application.