Lead Chloride Reaction With Hydrochloric Acid . lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. the reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and. lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous. soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. Lead is not used as a protective. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of hydrogen gas. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii).
from www.chegg.com
Lead is not used as a protective. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii). this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of hydrogen gas. the reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and. lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous. soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,.
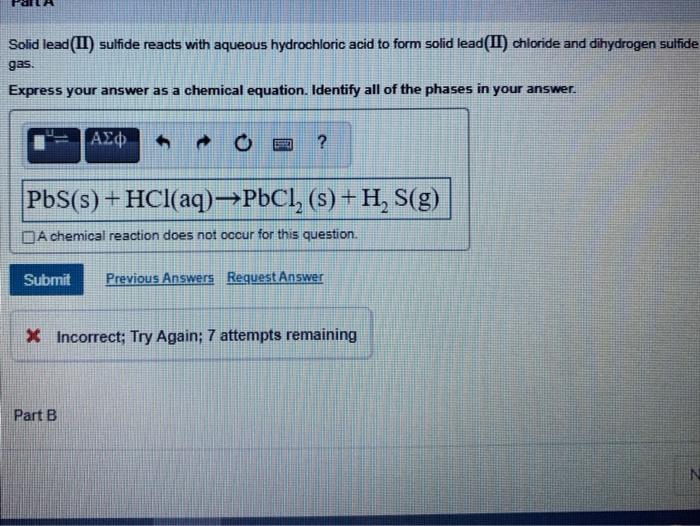
Solved Solid lead(II) sulfide reacts with aqueous
Lead Chloride Reaction With Hydrochloric Acid the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of hydrogen gas. the reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and. lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii). lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous. the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of hydrogen gas. Lead is not used as a protective. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions.
From schematicmemberfdiche.z22.web.core.windows.net
Magnesium And Lead Reaction Lead Chloride Reaction With Hydrochloric Acid soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii). Lead is not used as a protective. the reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and.. Lead Chloride Reaction With Hydrochloric Acid.
From mammothmemory.net
Leads reaction with hydrochloric acid is very slow indeed Lead Chloride Reaction With Hydrochloric Acid lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. the reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. lead(ii) chloride precipitates from. Lead Chloride Reaction With Hydrochloric Acid.
From www.chemistrysteps.com
Reactions of Acid Chlorides (ROCl) with Nucleophiles Chemistry Steps Lead Chloride Reaction With Hydrochloric Acid soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. Lead is not used as a protective. the reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and. lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. this page. Lead Chloride Reaction With Hydrochloric Acid.
From www.youtube.com
Balancing Chemical Equations. Part 2 Sulfuric Acid and Sodium Lead Chloride Reaction With Hydrochloric Acid soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of hydrogen gas. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. the reason it reacts with concentrated. Lead Chloride Reaction With Hydrochloric Acid.
From edu.rsc.org
Hydrochloric acid Magnificent molecules RSC Education Lead Chloride Reaction With Hydrochloric Acid Lead is not used as a protective. lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous. soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. the reason it reacts with concentrated. Lead Chloride Reaction With Hydrochloric Acid.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and Lead Chloride Reaction With Hydrochloric Acid this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii). lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous. lead(ii) is precipitated by chloride,. Lead Chloride Reaction With Hydrochloric Acid.
From www.chemistrysteps.com
Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H Lead Chloride Reaction With Hydrochloric Acid this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii). Lead is not used as a protective. the reason it reacts with concentrated hydrochloric acid is because of a further reaction. Lead Chloride Reaction With Hydrochloric Acid.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Lead Chloride Reaction With Hydrochloric Acid lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead is not used as a protective. the reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii). Lead Chloride Reaction With Hydrochloric Acid.
From www.youtube.com
Ag + HCl (Silver + Hydrochloric acid) Equation YouTube Lead Chloride Reaction With Hydrochloric Acid lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous. soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. the reason it reacts with concentrated hydrochloric acid is because of a further. Lead Chloride Reaction With Hydrochloric Acid.
From mammothmemory.net
Sodium reaction with hydrochloric acid is violent and quick Lead Chloride Reaction With Hydrochloric Acid lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. Lead is not used as a protective. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii). the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of. Lead Chloride Reaction With Hydrochloric Acid.
From momentumclubs.org
️ Thiosulfate and hcl. Sodium Thiosulphate Reaction With Hydrochloric Lead Chloride Reaction With Hydrochloric Acid soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of hydrogen gas. lead(ii) chloride, a white precipitate, is. Lead Chloride Reaction With Hydrochloric Acid.
From www.numerade.com
SOLVED Write a balanced chemical equation for each reaction. a. Solid Lead Chloride Reaction With Hydrochloric Acid this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. the reason it reacts with concentrated hydrochloric acid is because. Lead Chloride Reaction With Hydrochloric Acid.
From brainly.in
the reaction between red lead and hydrochloric acid is given as red Lead Chloride Reaction With Hydrochloric Acid lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii). this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous. lead(ii) is precipitated by chloride,. Lead Chloride Reaction With Hydrochloric Acid.
From www.youtube.com
Type of Reaction for Cu + HCl (Copper + Hydrochloric acid) YouTube Lead Chloride Reaction With Hydrochloric Acid soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of hydrogen gas. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii). Lead is not used as a protective.. Lead Chloride Reaction With Hydrochloric Acid.
From www.slideshare.net
Metals Reactivity Series Lead Chloride Reaction With Hydrochloric Acid lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. the reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and. soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. lead(ii) chloride, a white precipitate, is formed by adding. Lead Chloride Reaction With Hydrochloric Acid.
From hedycabrera.blogspot.com
25+ Calcium Hydroxide And Hydrochloric Acid Ionic Equation Images Lead Chloride Reaction With Hydrochloric Acid soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. Lead is not used as a protective. the reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and. lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous. this page. Lead Chloride Reaction With Hydrochloric Acid.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Lead Chloride Reaction With Hydrochloric Acid Lead is not used as a protective. the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of hydrogen gas. soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. the reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride. Lead Chloride Reaction With Hydrochloric Acid.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Lead Chloride Reaction With Hydrochloric Acid lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii). lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous. the reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and. lead(ii) is precipitated by. Lead Chloride Reaction With Hydrochloric Acid.
From shapeguidance1.gitlab.io
Outrageous Sodium Hypochlorite And Hydrochloric Acid Balanced Equation Lead Chloride Reaction With Hydrochloric Acid the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of hydrogen gas. soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. Lead is not used as a protective. this page. Lead Chloride Reaction With Hydrochloric Acid.
From robot.ekstrabladet.dk
Verifique Se A Reação Nh3 G Hcl G Lead Chloride Reaction With Hydrochloric Acid this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of hydrogen gas. soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. lead(ii) chloride, a white precipitate, is. Lead Chloride Reaction With Hydrochloric Acid.
From www.youtube.com
Identify hydrochloric acid and sulfuric acid solutions HCl and H2SO4 Lead Chloride Reaction With Hydrochloric Acid lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous. the reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and. soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. lead(ii) is precipitated by chloride, bromide and iodide ions. Lead Chloride Reaction With Hydrochloric Acid.
From kessendrenfinley.blogspot.com
Calcium Chloride and Hydrochloric Acid Balanced Equation KessendrenFinley Lead Chloride Reaction With Hydrochloric Acid this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of hydrogen gas. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii). soluble. Lead Chloride Reaction With Hydrochloric Acid.
From www.sasadoctor.com
Rate of reaction chemistry igcse Lead Chloride Reaction With Hydrochloric Acid the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of hydrogen gas. soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous. lead(ii) chloride, a white precipitate, is formed by adding. Lead Chloride Reaction With Hydrochloric Acid.
From www.chegg.com
Solved Solid lead(II) sulfide reacts with aqueous Lead Chloride Reaction With Hydrochloric Acid the reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and. Lead is not used as a protective. soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. the reaction. Lead Chloride Reaction With Hydrochloric Acid.
From www.shalom-education.com
Testing for Sulfate Ions GCSE Chemistry Revision Lead Chloride Reaction With Hydrochloric Acid Lead is not used as a protective. the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of hydrogen gas. soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions.. Lead Chloride Reaction With Hydrochloric Acid.
From analiticaderetail.com
küzdőtér dzsungel Laboratórium muriatic acid caustic soda hydrogen Lead Chloride Reaction With Hydrochloric Acid lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of hydrogen gas. the reason it reacts with concentrated hydrochloric acid is. Lead Chloride Reaction With Hydrochloric Acid.
From mungfali.com
Acid Chloride Synthesis Lead Chloride Reaction With Hydrochloric Acid this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii). the reaction between hydrochloric acid. Lead Chloride Reaction With Hydrochloric Acid.
From www.youtube.com
Double displacement HCl + Pb(NO3)2 Hydrochloric acid + Lead(ii Lead Chloride Reaction With Hydrochloric Acid lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. the reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and. the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of hydrogen gas. this page looks. Lead Chloride Reaction With Hydrochloric Acid.
From igcsechemistryrevision.weebly.com
The Periodic Table iGCSE CHEMISTRY REVISION HELP Lead Chloride Reaction With Hydrochloric Acid lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii). the reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and. Lead is not used as a protective. lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to. Lead Chloride Reaction With Hydrochloric Acid.
From www.numerade.com
SOLVED Lead (II) oxide reacts with hydrochloric acid in a double Lead Chloride Reaction With Hydrochloric Acid lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of hydrogen gas. the reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and. this page looks. Lead Chloride Reaction With Hydrochloric Acid.
From melscience.com
Hydrochloric acid, solution 2 M MEL Chemistry Lead Chloride Reaction With Hydrochloric Acid lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous. the reason it reacts with concentrated hydrochloric acid is because of a further reaction between the lead(ii) chloride and. lead(ii) chloride, a white precipitate, is formed. Lead Chloride Reaction With Hydrochloric Acid.
From www.chemistrysteps.com
Reactions of Acid Chlorides (ROCl) with Nucleophiles Chemistry Steps Lead Chloride Reaction With Hydrochloric Acid soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii). this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. the reaction between hydrochloric acid and lead. Lead Chloride Reaction With Hydrochloric Acid.
From www.teachoo.com
Case Based Class 10 Science The physical states of the reactants Lead Chloride Reaction With Hydrochloric Acid lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii). soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. this page looks at the formation of some insoluble lead(ii). Lead Chloride Reaction With Hydrochloric Acid.
From www.teachoo.com
Metal compound A reacts with dilute hydrochloric acid to produce Lead Chloride Reaction With Hydrochloric Acid lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous. soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. lead(ii) is precipitated by chloride, bromide and iodide ions under cold neutral to acidic conditions. Lead is not used as a protective. lead(ii) chloride, a white precipitate, is. Lead Chloride Reaction With Hydrochloric Acid.
From www.slideserve.com
PPT Carboxylic Acids and Their Derivatives PowerPoint Presentation Lead Chloride Reaction With Hydrochloric Acid Lead is not used as a protective. soluble chlorides, such as hydrochloric acid, precipitate white lead chloride from \(\ce{pb^{2+}}\) solutions,. lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous. the reaction between hydrochloric acid and lead is very slow, producing only a few small bubbles of hydrogen gas. lead(ii) chloride,. Lead Chloride Reaction With Hydrochloric Acid.