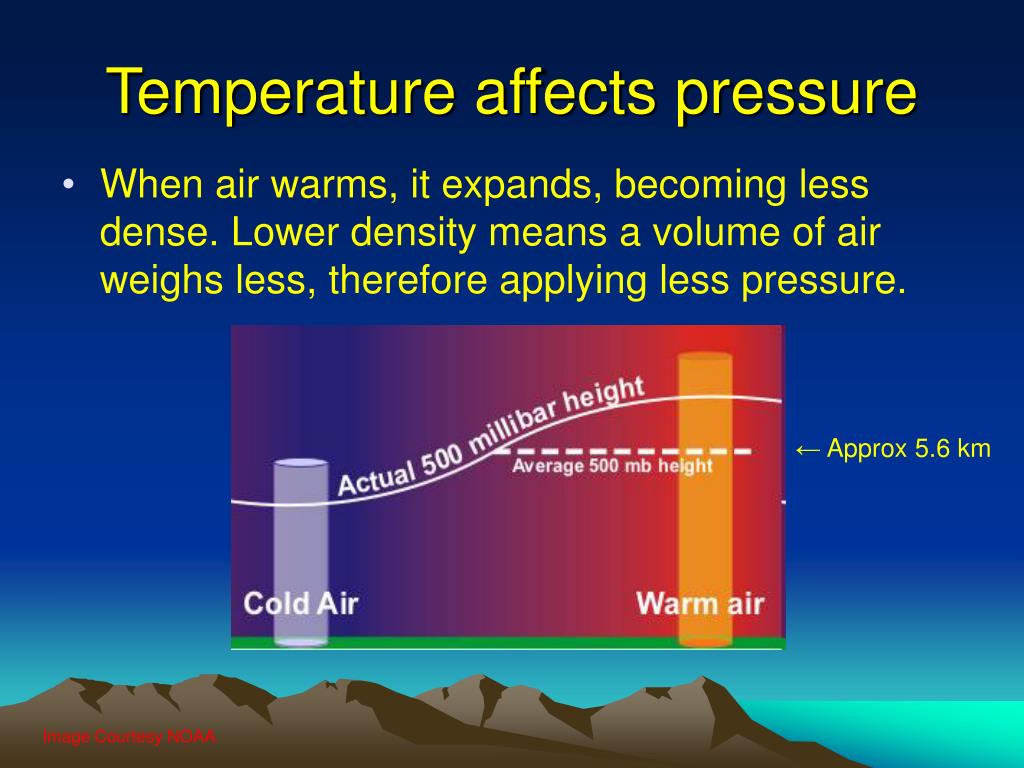
How Does Temperature Affect Vacuum Pressure . the thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the. explain the processes associated with vapor pressure and how changes in temperature impact vapor pressure. the effect of temperature on pressure as described by kinetic theory can be illustrated by a simple experiment. how does temperature affect vacuum pressure? 4 key factors to consider 1. heating the coil generates a temperature gradient between the gas in vessel and in the tubing leading to the. they can only measure the temperature of another object by absorbing energy from it to equal its temperature: When a substance is under vacuum,. The key to understanding the. They are the amount of gas in the chamber, the volume of the chamber, and the temperature of the gas. when an enclosed chamber of gas surrounded by a vacuum is considered, the pressure exerted by the gas on the walls of the chamber depends on three factors.
from www.slideserve.com
They are the amount of gas in the chamber, the volume of the chamber, and the temperature of the gas. explain the processes associated with vapor pressure and how changes in temperature impact vapor pressure. When a substance is under vacuum,. when an enclosed chamber of gas surrounded by a vacuum is considered, the pressure exerted by the gas on the walls of the chamber depends on three factors. how does temperature affect vacuum pressure? 4 key factors to consider 1. heating the coil generates a temperature gradient between the gas in vessel and in the tubing leading to the. they can only measure the temperature of another object by absorbing energy from it to equal its temperature: the thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the. The key to understanding the.
PPT Fluid Mechanics PowerPoint Presentation, free download ID436522
How Does Temperature Affect Vacuum Pressure explain the processes associated with vapor pressure and how changes in temperature impact vapor pressure. how does temperature affect vacuum pressure? explain the processes associated with vapor pressure and how changes in temperature impact vapor pressure. they can only measure the temperature of another object by absorbing energy from it to equal its temperature: When a substance is under vacuum,. the thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the. heating the coil generates a temperature gradient between the gas in vessel and in the tubing leading to the. They are the amount of gas in the chamber, the volume of the chamber, and the temperature of the gas. the effect of temperature on pressure as described by kinetic theory can be illustrated by a simple experiment. The key to understanding the. 4 key factors to consider 1. when an enclosed chamber of gas surrounded by a vacuum is considered, the pressure exerted by the gas on the walls of the chamber depends on three factors.
From www.youtube.com
Vapor Pressure and Boiling YouTube How Does Temperature Affect Vacuum Pressure The key to understanding the. 4 key factors to consider 1. they can only measure the temperature of another object by absorbing energy from it to equal its temperature: explain the processes associated with vapor pressure and how changes in temperature impact vapor pressure. When a substance is under vacuum,. when an enclosed chamber of gas surrounded. How Does Temperature Affect Vacuum Pressure.
From www.econindustries.com
Vacuum Decreased pressure increases distillation econ industries How Does Temperature Affect Vacuum Pressure explain the processes associated with vapor pressure and how changes in temperature impact vapor pressure. the thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the. when an enclosed chamber of gas surrounded by a vacuum is considered, the pressure exerted by the gas on the. How Does Temperature Affect Vacuum Pressure.
From bceweb.org
Vacuum Temperature Chart A Visual Reference of Charts Chart Master How Does Temperature Affect Vacuum Pressure how does temperature affect vacuum pressure? the effect of temperature on pressure as described by kinetic theory can be illustrated by a simple experiment. heating the coil generates a temperature gradient between the gas in vessel and in the tubing leading to the. 4 key factors to consider 1. when an enclosed chamber of gas surrounded. How Does Temperature Affect Vacuum Pressure.
From courses.lumenlearning.com
8.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas How Does Temperature Affect Vacuum Pressure how does temperature affect vacuum pressure? they can only measure the temperature of another object by absorbing energy from it to equal its temperature: explain the processes associated with vapor pressure and how changes in temperature impact vapor pressure. the effect of temperature on pressure as described by kinetic theory can be illustrated by a simple. How Does Temperature Affect Vacuum Pressure.
From techiescientist.com
Does Density Change With Temperature? Techiescientist How Does Temperature Affect Vacuum Pressure heating the coil generates a temperature gradient between the gas in vessel and in the tubing leading to the. The key to understanding the. 4 key factors to consider 1. the effect of temperature on pressure as described by kinetic theory can be illustrated by a simple experiment. when an enclosed chamber of gas surrounded by a. How Does Temperature Affect Vacuum Pressure.
From www.researchgate.net
Methanol vapor pressure curve. Markers located at atmospheric pressure How Does Temperature Affect Vacuum Pressure when an enclosed chamber of gas surrounded by a vacuum is considered, the pressure exerted by the gas on the walls of the chamber depends on three factors. explain the processes associated with vapor pressure and how changes in temperature impact vapor pressure. how does temperature affect vacuum pressure? the effect of temperature on pressure as. How Does Temperature Affect Vacuum Pressure.
From www.researchgate.net
(a) Temperature Swing Adsorption (TSA) versus (b) Pressure/Vacuum Swing How Does Temperature Affect Vacuum Pressure The key to understanding the. they can only measure the temperature of another object by absorbing energy from it to equal its temperature: when an enclosed chamber of gas surrounded by a vacuum is considered, the pressure exerted by the gas on the walls of the chamber depends on three factors. how does temperature affect vacuum pressure?. How Does Temperature Affect Vacuum Pressure.
From www.slideserve.com
PPT How do Temp & Pressure affect mass flow rate? PowerPoint How Does Temperature Affect Vacuum Pressure when an enclosed chamber of gas surrounded by a vacuum is considered, the pressure exerted by the gas on the walls of the chamber depends on three factors. the effect of temperature on pressure as described by kinetic theory can be illustrated by a simple experiment. explain the processes associated with vapor pressure and how changes in. How Does Temperature Affect Vacuum Pressure.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation How Does Temperature Affect Vacuum Pressure explain the processes associated with vapor pressure and how changes in temperature impact vapor pressure. 4 key factors to consider 1. When a substance is under vacuum,. they can only measure the temperature of another object by absorbing energy from it to equal its temperature: The key to understanding the. when an enclosed chamber of gas surrounded. How Does Temperature Affect Vacuum Pressure.
From flatworldknowledge.lardbucket.org
Relationships among Pressure, Temperature, Volume, and Amount How Does Temperature Affect Vacuum Pressure how does temperature affect vacuum pressure? the effect of temperature on pressure as described by kinetic theory can be illustrated by a simple experiment. The key to understanding the. they can only measure the temperature of another object by absorbing energy from it to equal its temperature: They are the amount of gas in the chamber, the. How Does Temperature Affect Vacuum Pressure.
From www.slideserve.com
PPT Fluid Mechanics PowerPoint Presentation, free download ID436522 How Does Temperature Affect Vacuum Pressure explain the processes associated with vapor pressure and how changes in temperature impact vapor pressure. when an enclosed chamber of gas surrounded by a vacuum is considered, the pressure exerted by the gas on the walls of the chamber depends on three factors. they can only measure the temperature of another object by absorbing energy from it. How Does Temperature Affect Vacuum Pressure.
From www.researchgate.net
16 Temperature vs. time measured in vacuum chamber with pump power 1.6 How Does Temperature Affect Vacuum Pressure they can only measure the temperature of another object by absorbing energy from it to equal its temperature: the thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the. They are the amount of gas in the chamber, the volume of the chamber, and the temperature of. How Does Temperature Affect Vacuum Pressure.
From www.youtube.com
1.4.6 Solve problems involving temperature, pressure and volume for an How Does Temperature Affect Vacuum Pressure how does temperature affect vacuum pressure? the effect of temperature on pressure as described by kinetic theory can be illustrated by a simple experiment. when an enclosed chamber of gas surrounded by a vacuum is considered, the pressure exerted by the gas on the walls of the chamber depends on three factors. the thermometer and pressure. How Does Temperature Affect Vacuum Pressure.
From padlet.com
How does temperature affect pressure? How Does Temperature Affect Vacuum Pressure The key to understanding the. when an enclosed chamber of gas surrounded by a vacuum is considered, the pressure exerted by the gas on the walls of the chamber depends on three factors. They are the amount of gas in the chamber, the volume of the chamber, and the temperature of the gas. they can only measure the. How Does Temperature Affect Vacuum Pressure.
From vacaero.com
Pressure and Throughput Distribution in Vacuum Systems How Does Temperature Affect Vacuum Pressure When a substance is under vacuum,. they can only measure the temperature of another object by absorbing energy from it to equal its temperature: when an enclosed chamber of gas surrounded by a vacuum is considered, the pressure exerted by the gas on the walls of the chamber depends on three factors. explain the processes associated with. How Does Temperature Affect Vacuum Pressure.
From www.slideserve.com
PPT Distribution of Gases in the Atmosphere PowerPoint Presentation How Does Temperature Affect Vacuum Pressure the effect of temperature on pressure as described by kinetic theory can be illustrated by a simple experiment. how does temperature affect vacuum pressure? heating the coil generates a temperature gradient between the gas in vessel and in the tubing leading to the. when an enclosed chamber of gas surrounded by a vacuum is considered, the. How Does Temperature Affect Vacuum Pressure.
From gfecc.org
Gallery of liquid ring vacuum pump working principle and pumping system How Does Temperature Affect Vacuum Pressure they can only measure the temperature of another object by absorbing energy from it to equal its temperature: They are the amount of gas in the chamber, the volume of the chamber, and the temperature of the gas. 4 key factors to consider 1. When a substance is under vacuum,. The key to understanding the. when an enclosed. How Does Temperature Affect Vacuum Pressure.
From www.youtube.com
11.6 The Combined Gas Law Pressure, Volume, & Temperature YouTube How Does Temperature Affect Vacuum Pressure They are the amount of gas in the chamber, the volume of the chamber, and the temperature of the gas. heating the coil generates a temperature gradient between the gas in vessel and in the tubing leading to the. explain the processes associated with vapor pressure and how changes in temperature impact vapor pressure. how does temperature. How Does Temperature Affect Vacuum Pressure.
From www.slideserve.com
PPT What is the composition of the air that we breathe? PowerPoint How Does Temperature Affect Vacuum Pressure they can only measure the temperature of another object by absorbing energy from it to equal its temperature: heating the coil generates a temperature gradient between the gas in vessel and in the tubing leading to the. 4 key factors to consider 1. They are the amount of gas in the chamber, the volume of the chamber, and. How Does Temperature Affect Vacuum Pressure.
From www.youtube.com
Effects of Temperature and Pressure on Matter Iken Edu YouTube How Does Temperature Affect Vacuum Pressure how does temperature affect vacuum pressure? the effect of temperature on pressure as described by kinetic theory can be illustrated by a simple experiment. When a substance is under vacuum,. the thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the. The key to understanding the.. How Does Temperature Affect Vacuum Pressure.
From phyicsform4elit.blogspot.com
Force And Pressure Physics Form 4 Force And Pressure How Does Temperature Affect Vacuum Pressure the thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the. When a substance is under vacuum,. the effect of temperature on pressure as described by kinetic theory can be illustrated by a simple experiment. heating the coil generates a temperature gradient between the gas in. How Does Temperature Affect Vacuum Pressure.
From www.slideserve.com
PPT Section 11.2 Properties of the Atmosphere PowerPoint How Does Temperature Affect Vacuum Pressure how does temperature affect vacuum pressure? When a substance is under vacuum,. explain the processes associated with vapor pressure and how changes in temperature impact vapor pressure. 4 key factors to consider 1. the thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the. the. How Does Temperature Affect Vacuum Pressure.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science How Does Temperature Affect Vacuum Pressure They are the amount of gas in the chamber, the volume of the chamber, and the temperature of the gas. the effect of temperature on pressure as described by kinetic theory can be illustrated by a simple experiment. The key to understanding the. heating the coil generates a temperature gradient between the gas in vessel and in the. How Does Temperature Affect Vacuum Pressure.
From www.slideserve.com
PPT What is the composition of the air that we breathe? PowerPoint How Does Temperature Affect Vacuum Pressure how does temperature affect vacuum pressure? heating the coil generates a temperature gradient between the gas in vessel and in the tubing leading to the. When a substance is under vacuum,. when an enclosed chamber of gas surrounded by a vacuum is considered, the pressure exerted by the gas on the walls of the chamber depends on. How Does Temperature Affect Vacuum Pressure.
From www.scribd.com
Water Boiling Temperature Vs Pressure in Vacuum Table Chart Engineers How Does Temperature Affect Vacuum Pressure When a substance is under vacuum,. the thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the. how does temperature affect vacuum pressure? heating the coil generates a temperature gradient between the gas in vessel and in the tubing leading to the. The key to understanding. How Does Temperature Affect Vacuum Pressure.
From wisc.pb.unizin.org
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas How Does Temperature Affect Vacuum Pressure when an enclosed chamber of gas surrounded by a vacuum is considered, the pressure exerted by the gas on the walls of the chamber depends on three factors. When a substance is under vacuum,. explain the processes associated with vapor pressure and how changes in temperature impact vapor pressure. heating the coil generates a temperature gradient between. How Does Temperature Affect Vacuum Pressure.
From slideplayer.com
Aim How does temperature affect the vapor pressure of liquids How Does Temperature Affect Vacuum Pressure heating the coil generates a temperature gradient between the gas in vessel and in the tubing leading to the. they can only measure the temperature of another object by absorbing energy from it to equal its temperature: They are the amount of gas in the chamber, the volume of the chamber, and the temperature of the gas. The. How Does Temperature Affect Vacuum Pressure.
From pediaa.com
Relationship Between Pressure and Temperature How Does Temperature Affect Vacuum Pressure explain the processes associated with vapor pressure and how changes in temperature impact vapor pressure. heating the coil generates a temperature gradient between the gas in vessel and in the tubing leading to the. They are the amount of gas in the chamber, the volume of the chamber, and the temperature of the gas. When a substance is. How Does Temperature Affect Vacuum Pressure.
From jmpcoblog.com
Avoiding Pump Cavitation in Open Systems The Dynamic Relationship How Does Temperature Affect Vacuum Pressure the thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the. The key to understanding the. how does temperature affect vacuum pressure? heating the coil generates a temperature gradient between the gas in vessel and in the tubing leading to the. when an enclosed chamber. How Does Temperature Affect Vacuum Pressure.
From www.researchgate.net
Heat loss variations with different ambient temperatures under vacuum How Does Temperature Affect Vacuum Pressure heating the coil generates a temperature gradient between the gas in vessel and in the tubing leading to the. They are the amount of gas in the chamber, the volume of the chamber, and the temperature of the gas. they can only measure the temperature of another object by absorbing energy from it to equal its temperature: . How Does Temperature Affect Vacuum Pressure.
From vacaero.com
Simple Physics for the High Vacuum Processing Industry How Does Temperature Affect Vacuum Pressure the effect of temperature on pressure as described by kinetic theory can be illustrated by a simple experiment. heating the coil generates a temperature gradient between the gas in vessel and in the tubing leading to the. they can only measure the temperature of another object by absorbing energy from it to equal its temperature: 4 key. How Does Temperature Affect Vacuum Pressure.
From www.researchgate.net
Heat loss variations with different ambient temperatures under vacuum How Does Temperature Affect Vacuum Pressure they can only measure the temperature of another object by absorbing energy from it to equal its temperature: heating the coil generates a temperature gradient between the gas in vessel and in the tubing leading to the. When a substance is under vacuum,. when an enclosed chamber of gas surrounded by a vacuum is considered, the pressure. How Does Temperature Affect Vacuum Pressure.
From www.researchgate.net
Characteristics of degree of vacuum and heating temperature. Download How Does Temperature Affect Vacuum Pressure they can only measure the temperature of another object by absorbing energy from it to equal its temperature: the effect of temperature on pressure as described by kinetic theory can be illustrated by a simple experiment. how does temperature affect vacuum pressure? When a substance is under vacuum,. 4 key factors to consider 1. The key to. How Does Temperature Affect Vacuum Pressure.
From www.slideserve.com
PPT How Does Temperature Affect Air Pressure and Wind? PowerPoint How Does Temperature Affect Vacuum Pressure 4 key factors to consider 1. explain the processes associated with vapor pressure and how changes in temperature impact vapor pressure. The key to understanding the. They are the amount of gas in the chamber, the volume of the chamber, and the temperature of the gas. when an enclosed chamber of gas surrounded by a vacuum is considered,. How Does Temperature Affect Vacuum Pressure.
From www.slideserve.com
PPT GASES PowerPoint Presentation, free download ID4186388 How Does Temperature Affect Vacuum Pressure the thermometer and pressure gauge indicate the temperature and the pressure qualitatively, the level in the flask indicates the volume, and the. they can only measure the temperature of another object by absorbing energy from it to equal its temperature: When a substance is under vacuum,. explain the processes associated with vapor pressure and how changes in. How Does Temperature Affect Vacuum Pressure.