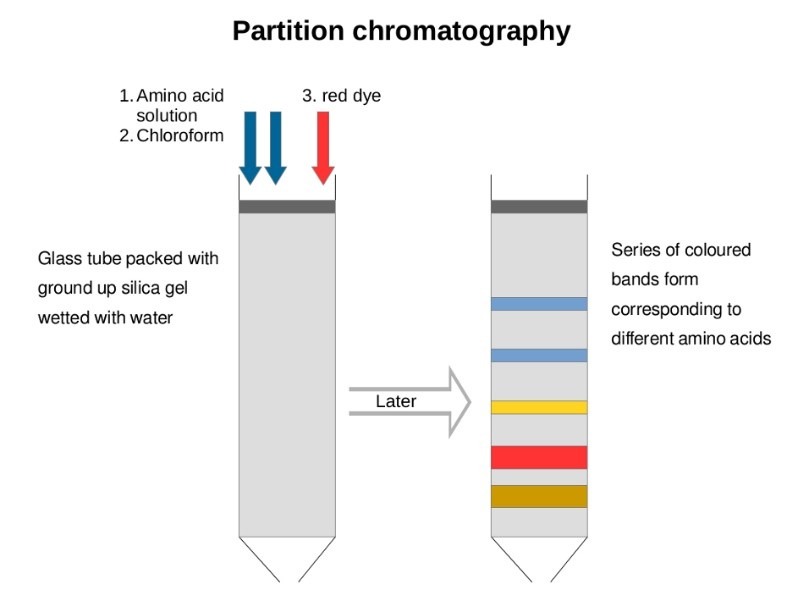
Paper Chromatography More Polar Mobile Phase . a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as descending mode is also. carrying out paper chromatography. if the eluent is made to be moderately polar, polar compounds are then more attracted to the mobile phase, causing the equilibrium to change such that the compound spends more time in the mobile phase, resulting in a higher \(r_f\). mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more. Chromatography is used to separate mixtures of substances into their. performing a chromatographic experiment is basically a three‐step process: generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will tend to.
from mungfali.com
a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as descending mode is also. if the eluent is made to be moderately polar, polar compounds are then more attracted to the mobile phase, causing the equilibrium to change such that the compound spends more time in the mobile phase, resulting in a higher \(r_f\). generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will tend to. carrying out paper chromatography. Chromatography is used to separate mixtures of substances into their. performing a chromatographic experiment is basically a three‐step process: mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more.
Paper Chromatography Labelled Diagram
Paper Chromatography More Polar Mobile Phase carrying out paper chromatography. performing a chromatographic experiment is basically a three‐step process: if the eluent is made to be moderately polar, polar compounds are then more attracted to the mobile phase, causing the equilibrium to change such that the compound spends more time in the mobile phase, resulting in a higher \(r_f\). a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as descending mode is also. Chromatography is used to separate mixtures of substances into their. mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more. generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will tend to. carrying out paper chromatography.
From www.slideserve.com
PPT What Is HPLC? PowerPoint Presentation, free download ID4713396 Paper Chromatography More Polar Mobile Phase mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more. performing a chromatographic experiment is basically a three‐step process: a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as descending mode is also. Chromatography is. Paper Chromatography More Polar Mobile Phase.
From www.slideserve.com
PPT Drugs & Crime PowerPoint Presentation ID6645712 Paper Chromatography More Polar Mobile Phase Chromatography is used to separate mixtures of substances into their. performing a chromatographic experiment is basically a three‐step process: generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will tend to. mixtures are separated because some components will be more attracted to the stationary phase (and stick to it). Paper Chromatography More Polar Mobile Phase.
From www.slideshare.net
Planar Chromatography Paper Chromatography More Polar Mobile Phase a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as descending mode is also. performing a chromatographic experiment is basically a three‐step process: if the eluent is made to be moderately polar, polar compounds are then more attracted to the mobile phase, causing the equilibrium to change such that. Paper Chromatography More Polar Mobile Phase.
From www.numerade.com
Scenario 5 Components of a Mixture Description Paper chromatography Paper Chromatography More Polar Mobile Phase generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will tend to. mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more. if the eluent is made to be moderately polar, polar compounds are then. Paper Chromatography More Polar Mobile Phase.
From chem.libretexts.org
2 Paper Chromatography of Gel Ink Pens (Experiment) Chemistry LibreTexts Paper Chromatography More Polar Mobile Phase generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will tend to. mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more. performing a chromatographic experiment is basically a three‐step process: if the eluent. Paper Chromatography More Polar Mobile Phase.
From www.slideserve.com
PPT Chapter 7 Drugs PowerPoint Presentation ID5611118 Paper Chromatography More Polar Mobile Phase Chromatography is used to separate mixtures of substances into their. mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more. performing a chromatographic experiment is basically a three‐step process: generally, if the solvent (mobile phase) is more polar than the stationary phase, the. Paper Chromatography More Polar Mobile Phase.
From www.numerade.com
SOLVED Student Activity Chromatography Separation of Photopigments Paper Chromatography More Polar Mobile Phase Chromatography is used to separate mixtures of substances into their. mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more. performing a chromatographic experiment is basically a three‐step process: generally, if the solvent (mobile phase) is more polar than the stationary phase, the. Paper Chromatography More Polar Mobile Phase.
From hubpages.com
What Is Paper Chromatography Principle, Types, & Uses Owlcation Paper Chromatography More Polar Mobile Phase performing a chromatographic experiment is basically a three‐step process: carrying out paper chromatography. if the eluent is made to be moderately polar, polar compounds are then more attracted to the mobile phase, causing the equilibrium to change such that the compound spends more time in the mobile phase, resulting in a higher \(r_f\). mixtures are separated. Paper Chromatography More Polar Mobile Phase.
From namrataheda.blogspot.com
B for Biology Chromatography Paper Chromatography Paper Chromatography More Polar Mobile Phase carrying out paper chromatography. if the eluent is made to be moderately polar, polar compounds are then more attracted to the mobile phase, causing the equilibrium to change such that the compound spends more time in the mobile phase, resulting in a higher \(r_f\). generally, if the solvent (mobile phase) is more polar than the stationary phase,. Paper Chromatography More Polar Mobile Phase.
From www.slideserve.com
PPT What is Chromatography? PowerPoint Presentation, free download Paper Chromatography More Polar Mobile Phase carrying out paper chromatography. generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will tend to. mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more. Chromatography is used to separate mixtures of substances into. Paper Chromatography More Polar Mobile Phase.
From owlcation.com
What Is Paper Chromatography and How Does It Work? Owlcation Paper Chromatography More Polar Mobile Phase generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will tend to. Chromatography is used to separate mixtures of substances into their. if the eluent is made to be moderately polar, polar compounds are then more attracted to the mobile phase, causing the equilibrium to change such that the compound. Paper Chromatography More Polar Mobile Phase.
From www.yaclass.in
Paper chromatography — lesson. Science State Board, Class 9. Paper Chromatography More Polar Mobile Phase carrying out paper chromatography. Chromatography is used to separate mixtures of substances into their. performing a chromatographic experiment is basically a three‐step process: if the eluent is made to be moderately polar, polar compounds are then more attracted to the mobile phase, causing the equilibrium to change such that the compound spends more time in the mobile. Paper Chromatography More Polar Mobile Phase.
From www.youtube.com
Molecular Polarity used in Chromatography YouTube Paper Chromatography More Polar Mobile Phase Chromatography is used to separate mixtures of substances into their. generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will tend to. mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more. if the eluent. Paper Chromatography More Polar Mobile Phase.
From microbenotes.com
Paper Chromatography Definition, Types, Principle, Steps, Uses Paper Chromatography More Polar Mobile Phase Chromatography is used to separate mixtures of substances into their. a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as descending mode is also. carrying out paper chromatography. generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will tend to. . Paper Chromatography More Polar Mobile Phase.
From slidetodoc.com
Colors in Chemistry Learning Polarity with Paper Chromatography Paper Chromatography More Polar Mobile Phase Chromatography is used to separate mixtures of substances into their. mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more. a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as descending mode is also. generally,. Paper Chromatography More Polar Mobile Phase.
From www.youtube.com
Paper Chromatography Intro & Theory YouTube Paper Chromatography More Polar Mobile Phase carrying out paper chromatography. a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as descending mode is also. mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more. generally, if the solvent (mobile phase). Paper Chromatography More Polar Mobile Phase.
From www.numerade.com
SOLVED 2. What is the mobile phase used for the paper chromatography Paper Chromatography More Polar Mobile Phase carrying out paper chromatography. mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more. performing a chromatographic experiment is basically a three‐step process: generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will tend. Paper Chromatography More Polar Mobile Phase.
From www.vrogue.co
What Is Paper Chromatography And How Does It Work Hig vrogue.co Paper Chromatography More Polar Mobile Phase mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more. generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will tend to. if the eluent is made to be moderately polar, polar compounds are then. Paper Chromatography More Polar Mobile Phase.
From dxoqqejay.blob.core.windows.net
How To Analyze Chromatography Paper at Dinah Humphrey blog Paper Chromatography More Polar Mobile Phase a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as descending mode is also. Chromatography is used to separate mixtures of substances into their. generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will tend to. performing a chromatographic experiment is. Paper Chromatography More Polar Mobile Phase.
From www.slideserve.com
PPT Chromatography PowerPoint Presentation, free download ID542840 Paper Chromatography More Polar Mobile Phase if the eluent is made to be moderately polar, polar compounds are then more attracted to the mobile phase, causing the equilibrium to change such that the compound spends more time in the mobile phase, resulting in a higher \(r_f\). a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as. Paper Chromatography More Polar Mobile Phase.
From www.chemistrystudent.com
Chromatography (ALevel) ChemistryStudent Paper Chromatography More Polar Mobile Phase Chromatography is used to separate mixtures of substances into their. a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as descending mode is also. if the eluent is made to be moderately polar, polar compounds are then more attracted to the mobile phase, causing the equilibrium to change such that. Paper Chromatography More Polar Mobile Phase.
From mavink.com
Chromatography Phases Paper Chromatography More Polar Mobile Phase if the eluent is made to be moderately polar, polar compounds are then more attracted to the mobile phase, causing the equilibrium to change such that the compound spends more time in the mobile phase, resulting in a higher \(r_f\). carrying out paper chromatography. Chromatography is used to separate mixtures of substances into their. generally, if the. Paper Chromatography More Polar Mobile Phase.
From www.youtube.com
Introduction to Chromatography 2 Mobile Phase YouTube Paper Chromatography More Polar Mobile Phase Chromatography is used to separate mixtures of substances into their. if the eluent is made to be moderately polar, polar compounds are then more attracted to the mobile phase, causing the equilibrium to change such that the compound spends more time in the mobile phase, resulting in a higher \(r_f\). generally, if the solvent (mobile phase) is more. Paper Chromatography More Polar Mobile Phase.
From mungfali.com
Paper Chromatography Labelled Diagram Paper Chromatography More Polar Mobile Phase a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as descending mode is also. Chromatography is used to separate mixtures of substances into their. mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more. performing. Paper Chromatography More Polar Mobile Phase.
From chemistrytalk.org
Paper and ThinLayer Chromatography ChemTalk Paper Chromatography More Polar Mobile Phase generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will tend to. a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as descending mode is also. mixtures are separated because some components will be more attracted to the stationary phase (and. Paper Chromatography More Polar Mobile Phase.
From majorscienceandtechnology.blogspot.com
Classification of chromatography Major Science and technology Paper Chromatography More Polar Mobile Phase if the eluent is made to be moderately polar, polar compounds are then more attracted to the mobile phase, causing the equilibrium to change such that the compound spends more time in the mobile phase, resulting in a higher \(r_f\). generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will. Paper Chromatography More Polar Mobile Phase.
From thechemistrynotes.com
Paper Chromatography Paper Chromatography More Polar Mobile Phase generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will tend to. a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as descending mode is also. carrying out paper chromatography. if the eluent is made to be moderately polar, polar. Paper Chromatography More Polar Mobile Phase.
From www.slidemake.com
Stationary Mobile Phase And Application Of Gas Chromatography Presentation Paper Chromatography More Polar Mobile Phase Chromatography is used to separate mixtures of substances into their. a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as descending mode is also. mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more. performing. Paper Chromatography More Polar Mobile Phase.
From www.priyamstudycentre.com
Chromatography Techniques, Definition, Principle, Types Paper Chromatography More Polar Mobile Phase generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will tend to. Chromatography is used to separate mixtures of substances into their. performing a chromatographic experiment is basically a three‐step process: a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as. Paper Chromatography More Polar Mobile Phase.
From www.differencebetween.com
Difference Between Stationary and Mobile Phase Compare the Difference Paper Chromatography More Polar Mobile Phase Chromatography is used to separate mixtures of substances into their. if the eluent is made to be moderately polar, polar compounds are then more attracted to the mobile phase, causing the equilibrium to change such that the compound spends more time in the mobile phase, resulting in a higher \(r_f\). a few centimeters of the sheet are dipped. Paper Chromatography More Polar Mobile Phase.
From www.istockphoto.com
Vector Scientific Illustration Of Ascending Paper Chromatography An Paper Chromatography More Polar Mobile Phase performing a chromatographic experiment is basically a three‐step process: mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more. if the eluent is made to be moderately polar, polar compounds are then more attracted to the mobile phase, causing the equilibrium to change. Paper Chromatography More Polar Mobile Phase.
From ar.inspiredpencil.com
Paper Chromatography Diagram Stationary Phase Paper Chromatography More Polar Mobile Phase Chromatography is used to separate mixtures of substances into their. a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as descending mode is also. mixtures are separated because some components will be more attracted to the stationary phase (and stick to it) while some components will be more. carrying. Paper Chromatography More Polar Mobile Phase.
From www.youtube.com
Polarity Demonstration Paper Chromatography Video 2 YouTube Paper Chromatography More Polar Mobile Phase Chromatography is used to separate mixtures of substances into their. carrying out paper chromatography. performing a chromatographic experiment is basically a three‐step process: a few centimeters of the sheet are dipped into the mobile phase which then ascends (or descends, as descending mode is also. mixtures are separated because some components will be more attracted to. Paper Chromatography More Polar Mobile Phase.
From www.slideserve.com
PPT BASED ON POLARITY PowerPoint Presentation, free download ID1430840 Paper Chromatography More Polar Mobile Phase if the eluent is made to be moderately polar, polar compounds are then more attracted to the mobile phase, causing the equilibrium to change such that the compound spends more time in the mobile phase, resulting in a higher \(r_f\). performing a chromatographic experiment is basically a three‐step process: carrying out paper chromatography. generally, if the. Paper Chromatography More Polar Mobile Phase.
From exofmwamp.blob.core.windows.net
Paper Chromatography Class 12 Practical at Carolyn Brunson blog Paper Chromatography More Polar Mobile Phase Chromatography is used to separate mixtures of substances into their. performing a chromatographic experiment is basically a three‐step process: generally, if the solvent (mobile phase) is more polar than the stationary phase, the more polar compounds will tend to. if the eluent is made to be moderately polar, polar compounds are then more attracted to the mobile. Paper Chromatography More Polar Mobile Phase.