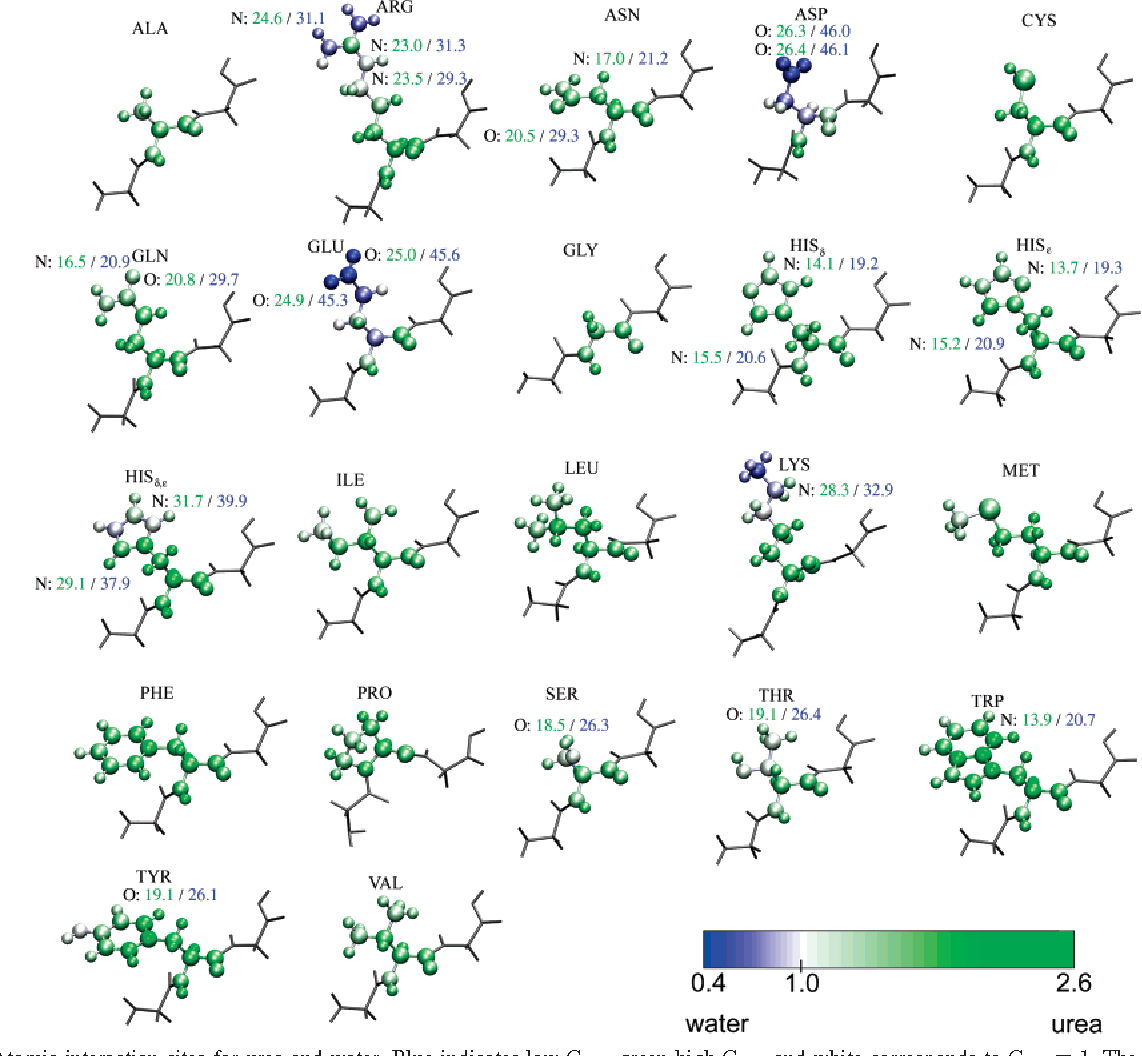
Protein Denaturation Urea . whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but conflicting features. by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. in particular, the simulations reveal a stepwise process, starting from a. by elucidating the interaction between urea, water, and the hydrophobic side chain of odpa, our study provides. solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic. chemical denaturation, with an agent such as urea, is one of the primary. the ability of urea to interact with both nonpolar and polar components of proteins was recognized early on as beneficial to.
from www.semanticscholar.org
in particular, the simulations reveal a stepwise process, starting from a. whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. the ability of urea to interact with both nonpolar and polar components of proteins was recognized early on as beneficial to. solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic. chemical denaturation, with an agent such as urea, is one of the primary. by elucidating the interaction between urea, water, and the hydrophobic side chain of odpa, our study provides. by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but conflicting features.
Figure 2 from Interaction of urea with amino acids implications for
Protein Denaturation Urea by elucidating the interaction between urea, water, and the hydrophobic side chain of odpa, our study provides. chemical denaturation, with an agent such as urea, is one of the primary. According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but conflicting features. whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. by elucidating the interaction between urea, water, and the hydrophobic side chain of odpa, our study provides. by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic. in particular, the simulations reveal a stepwise process, starting from a. the ability of urea to interact with both nonpolar and polar components of proteins was recognized early on as beneficial to.
From www.researchgate.net
(a) Schematic illustration of the urea?induced CP denaturation via Protein Denaturation Urea whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic. According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but conflicting features. in particular, the simulations reveal. Protein Denaturation Urea.
From www.pnas.org
Counteraction of ureainduced protein denaturation by trimethylamine N Protein Denaturation Urea chemical denaturation, with an agent such as urea, is one of the primary. by elucidating the interaction between urea, water, and the hydrophobic side chain of odpa, our study provides. whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. in particular, the simulations reveal a stepwise process,. Protein Denaturation Urea.
From www.researchgate.net
(PDF) Ureainduced denaturation of human calciumcalmodulin dependent Protein Denaturation Urea in particular, the simulations reveal a stepwise process, starting from a. According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but conflicting features. whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. by systematically varying urea polarity and quantifying. Protein Denaturation Urea.
From pubs.acs.org
Equilibrium Study of Protein Denaturation by Urea Journal of the Protein Denaturation Urea by elucidating the interaction between urea, water, and the hydrophobic side chain of odpa, our study provides. According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but conflicting features. by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein,. Protein Denaturation Urea.
From www.pnas.org
Counteraction of ureainduced protein denaturation by trimethylamine N Protein Denaturation Urea the ability of urea to interact with both nonpolar and polar components of proteins was recognized early on as beneficial to. According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but conflicting features. solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic. whereas urea−protein. Protein Denaturation Urea.
From www.researchgate.net
(PDF) Ammonium based stabilizers effectively counteract ureainduced Protein Denaturation Urea whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. by elucidating the interaction between urea, water, and the hydrophobic side chain of odpa, our study provides. chemical denaturation, with an agent such as urea, is one of the primary. According to the mechanism proposed here, the denaturation power. Protein Denaturation Urea.
From www.researchgate.net
Stability analysis of NRN1L.h.variants. Protein denaturation was Protein Denaturation Urea whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but conflicting features. chemical denaturation, with an agent such as urea, is one of the primary. the ability of urea to. Protein Denaturation Urea.
From pubs.acs.org
Interaction of Urea with Amino Acids Implications for UreaInduced Protein Denaturation Urea by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. by elucidating the interaction between urea, water, and the hydrophobic side chain of odpa, our study provides. chemical denaturation, with an agent such as urea, is one of the primary. in particular, the simulations reveal. Protein Denaturation Urea.
From pubs.acs.org
UreaMediated Protein Denaturation A Consensus View The Journal of Protein Denaturation Urea by elucidating the interaction between urea, water, and the hydrophobic side chain of odpa, our study provides. solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic. whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. the ability of urea to interact with both. Protein Denaturation Urea.
From www.youtube.com
DETECT PROTEIN UREA DENATURATION FEDERAL BOARD PRACTICALS CHEMISTRY Protein Denaturation Urea whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but. Protein Denaturation Urea.
From www.researchgate.net
(PDF) UreaMediated Protein Denaturation A Consensus View Protein Denaturation Urea by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. the ability of urea to interact with both nonpolar and polar components of proteins was recognized early on. Protein Denaturation Urea.
From dxoncrrss.blob.core.windows.net
Protein Denaturation Western Blot at Stephanie Lively blog Protein Denaturation Urea by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but conflicting features. in particular, the simulations reveal a stepwise process, starting from a. the ability of urea. Protein Denaturation Urea.
From www.researchgate.net
Effect of protein concentration on the urea unfolding curve of LS 5 nM Protein Denaturation Urea in particular, the simulations reveal a stepwise process, starting from a. by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. by elucidating the interaction between urea, water, and the hydrophobic side chain of odpa, our study provides. chemical denaturation, with an agent such as. Protein Denaturation Urea.
From byjus.com
Denaturation Of Proteins By Urea Enzymes Of Denatured Ph Value Protein Denaturation Urea chemical denaturation, with an agent such as urea, is one of the primary. by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. by elucidating the interaction. Protein Denaturation Urea.
From www.researchgate.net
(PDF) Native protein denaturation using urea Protein Denaturation Urea whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. chemical denaturation, with an agent such as urea, is one of the primary. According to the mechanism proposed. Protein Denaturation Urea.
From sciencing.com
How Does Urea Denature Proteins? Sciencing Protein Denaturation Urea According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but conflicting features. solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic. the ability of urea to interact with both nonpolar and polar components of proteins was recognized early on as beneficial to. by systematically. Protein Denaturation Urea.
From dokumen.tips
(PDF) On the Mechanism of UreaInduced Protein Denaturation310217 Protein Denaturation Urea the ability of urea to interact with both nonpolar and polar components of proteins was recognized early on as beneficial to. According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but conflicting features. by elucidating the interaction between urea, water, and the hydrophobic side chain of odpa, our study. Protein Denaturation Urea.
From www.researchgate.net
Schematic illustration of the urea induced CP denaturation via indirect Protein Denaturation Urea by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. chemical denaturation, with an agent such as urea, is one of the primary. by elucidating the interaction between urea, water, and the hydrophobic side chain of odpa, our study provides. solvation of the protein backbone. Protein Denaturation Urea.
From www.nutrisoil.com.au
"Deciphering Protein Mysteries Urea's Dual Role in Protein Protein Denaturation Urea by elucidating the interaction between urea, water, and the hydrophobic side chain of odpa, our study provides. by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but conflicting. Protein Denaturation Urea.
From www.semanticscholar.org
Figure 2 from Interaction of urea with amino acids implications for Protein Denaturation Urea by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. chemical denaturation, with an agent such as urea, is one of the primary. solvation of the protein. Protein Denaturation Urea.
From www.researchgate.net
(PDF) Pressure and UreaInduced Denaturation of Bovine Serum Albumin Protein Denaturation Urea in particular, the simulations reveal a stepwise process, starting from a. by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. chemical denaturation, with an agent such as urea, is one of the primary. by elucidating the interaction between urea, water, and the hydrophobic side. Protein Denaturation Urea.
From www.pnas.org
Urea denaturation by stronger dispersion interactions with proteins Protein Denaturation Urea the ability of urea to interact with both nonpolar and polar components of proteins was recognized early on as beneficial to. chemical denaturation, with an agent such as urea, is one of the primary. According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but conflicting features. solvation of. Protein Denaturation Urea.
From pubs.acs.org
Interaction of Urea with Amino Acids Implications for UreaInduced Protein Denaturation Urea by elucidating the interaction between urea, water, and the hydrophobic side chain of odpa, our study provides. solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic. in particular, the simulations reveal a stepwise process, starting from a. chemical denaturation, with an agent such as urea, is one of the primary. by. Protein Denaturation Urea.
From pubs.rsc.org
The shift in urea orientation at protein surfaces at low pH is Protein Denaturation Urea by elucidating the interaction between urea, water, and the hydrophobic side chain of odpa, our study provides. chemical denaturation, with an agent such as urea, is one of the primary. According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but conflicting features. whereas urea−protein hydrogen bonds do not. Protein Denaturation Urea.
From pubs.rsc.org
The shift in urea orientation at protein surfaces at low pH is Protein Denaturation Urea in particular, the simulations reveal a stepwise process, starting from a. the ability of urea to interact with both nonpolar and polar components of proteins was recognized early on as beneficial to. whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. by systematically varying urea polarity and. Protein Denaturation Urea.
From pubs.acs.org
Comment on “UreaMediated Protein Denaturation A Consensus View” The Protein Denaturation Urea solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic. whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. in particular, the simulations reveal a stepwise process, starting from a. the ability of urea to interact with both nonpolar and polar components of proteins. Protein Denaturation Urea.
From achs-prod.acs.org
Mechanical Insight into Resistance of Betaine to UreaInduced Protein Protein Denaturation Urea by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic. According to the mechanism proposed. Protein Denaturation Urea.
From www.researchgate.net
(PDF) Atomistic Mechanism of Protein Denaturation by Urea Protein Denaturation Urea by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. by elucidating the interaction between urea, water, and the hydrophobic side chain of odpa, our study provides. the ability of urea to interact with both nonpolar and polar components of proteins was recognized early on as. Protein Denaturation Urea.
From pubs.rsc.org
The shift in urea orientation at protein surfaces at low pH is Protein Denaturation Urea chemical denaturation, with an agent such as urea, is one of the primary. the ability of urea to interact with both nonpolar and polar components of proteins was recognized early on as beneficial to. whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. According to the mechanism proposed. Protein Denaturation Urea.
From www.researchgate.net
(PDF) Counteraction of ureainduced protein denaturation by Protein Denaturation Urea solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic. According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but conflicting features. by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. chemical denaturation,. Protein Denaturation Urea.
From www.researchgate.net
Normalized equilibrium urea denaturation curves measured by tyrosine Protein Denaturation Urea chemical denaturation, with an agent such as urea, is one of the primary. the ability of urea to interact with both nonpolar and polar components of proteins was recognized early on as beneficial to. in particular, the simulations reveal a stepwise process, starting from a. solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction. Protein Denaturation Urea.
From www.researchgate.net
(PDF) Mechanism of Protein Denaturation Partial Unfolding of the P22 Protein Denaturation Urea whereas urea−protein hydrogen bonds do not seem to drive the denaturation, they do contribute to the overall energetics. According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but conflicting features. chemical denaturation, with an agent such as urea, is one of the primary. solvation of the protein backbone. Protein Denaturation Urea.
From www.researchgate.net
Protein Denaturation Urea or high temperature? ResearchGate Protein Denaturation Urea solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic. chemical denaturation, with an agent such as urea, is one of the primary. According to the mechanism proposed here, the denaturation power of urea rests on its tradeoff between two essential but conflicting features. the ability of urea to interact with both nonpolar and. Protein Denaturation Urea.
From www.pnas.org
The molecular basis for the chemical denaturation of proteins by urea Protein Denaturation Urea solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic. in particular, the simulations reveal a stepwise process, starting from a. chemical denaturation, with an agent such as urea, is one of the primary. by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the. Protein Denaturation Urea.
From www.pnas.org
Counteraction of ureainduced protein denaturation by trimethylamine N Protein Denaturation Urea by systematically varying urea polarity and quantifying the interactions of the solvent molecules with all amino acids of the protein, the. the ability of urea to interact with both nonpolar and polar components of proteins was recognized early on as beneficial to. solvation of the protein backbone via hydrogen bonding, favorable electrostatic interaction with hydrophilic. by. Protein Denaturation Urea.