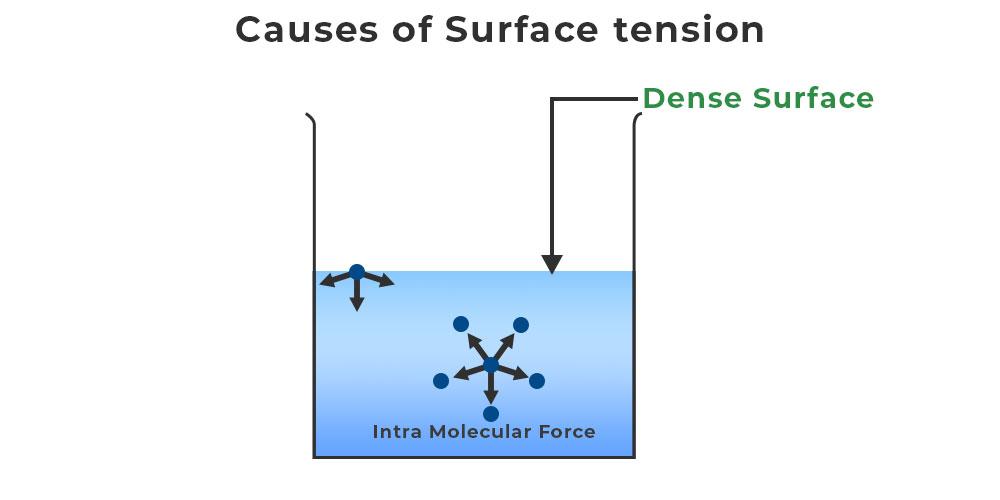
What If Water Has No Surface Tension . surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. In comparison, organic liquids, such as benzene and alcohols,. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water consists of one oxygen atom flanked by. the team measured surface tension in seawater ranging from room temperature to 90 degrees celsius and salinity levels ranging from pure water. you could experiment a bit with fluids that have lower surface tension than water and see how they react and then extrapolate. water has high surface tension, which can be explained by its polarity and hydrogen bonding. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes.
from upberi.com
water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. Water consists of one oxygen atom flanked by. the team measured surface tension in seawater ranging from room temperature to 90 degrees celsius and salinity levels ranging from pure water. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. In comparison, organic liquids, such as benzene and alcohols,. you could experiment a bit with fluids that have lower surface tension than water and see how they react and then extrapolate.
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023)
What If Water Has No Surface Tension water has high surface tension, which can be explained by its polarity and hydrogen bonding. you could experiment a bit with fluids that have lower surface tension than water and see how they react and then extrapolate. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. the team measured surface tension in seawater ranging from room temperature to 90 degrees celsius and salinity levels ranging from pure water. Water consists of one oxygen atom flanked by. water has high surface tension, which can be explained by its polarity and hydrogen bonding. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. In comparison, organic liquids, such as benzene and alcohols,. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f).
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 What If Water Has No Surface Tension surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. Water consists of one oxygen atom flanked by. water has high surface tension, which can be explained by its polarity and hydrogen bonding. water has a surface tension of 0.07275 joule per square metre. What If Water Has No Surface Tension.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) What If Water Has No Surface Tension the team measured surface tension in seawater ranging from room temperature to 90 degrees celsius and salinity levels ranging from pure water. water has high surface tension, which can be explained by its polarity and hydrogen bonding. if you look at the surface tension between water and water vapor as the critical point is approached, the surface. What If Water Has No Surface Tension.
From mirjamglessmer.com
Trying to understand surface tension Adventures in Oceanography and What If Water Has No Surface Tension if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. you could experiment a bit with fluids that have lower surface tension than water and see how they react and then extrapolate. water has high surface tension, which can be explained by its polarity and. What If Water Has No Surface Tension.
From www.slideserve.com
PPT FLUID PROPERTIES Chapter 2 PowerPoint Presentation, free download What If Water Has No Surface Tension the team measured surface tension in seawater ranging from room temperature to 90 degrees celsius and salinity levels ranging from pure water. you could experiment a bit with fluids that have lower surface tension than water and see how they react and then extrapolate. In comparison, organic liquids, such as benzene and alcohols,. water has high surface. What If Water Has No Surface Tension.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical What If Water Has No Surface Tension the team measured surface tension in seawater ranging from room temperature to 90 degrees celsius and salinity levels ranging from pure water. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. In comparison, organic liquids, such as benzene and alcohols,. water has high surface. What If Water Has No Surface Tension.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts What If Water Has No Surface Tension surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. you could experiment a bit with fluids that have lower surface tension than water and see how they react and then extrapolate. the team measured surface tension in seawater ranging from room temperature to. What If Water Has No Surface Tension.
From www.slideserve.com
PPT CEE 210 Environmental Biology for Engineers PowerPoint What If Water Has No Surface Tension if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. Water consists of one oxygen atom flanked by. In comparison, organic liquids, such as benzene and alcohols,. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension in. What If Water Has No Surface Tension.
From ifunny.co
Swimmers just before the water’s surface tension is broken What if What If Water Has No Surface Tension you could experiment a bit with fluids that have lower surface tension than water and see how they react and then extrapolate. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. surface tension of water can cause things to float which are denser. What If Water Has No Surface Tension.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog What If Water Has No Surface Tension surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. Water consists of one oxygen atom flanked by. In comparison, organic liquids, such as benzene and alcohols,. you could experiment a bit with fluids that have lower surface tension than water and see how they react. What If Water Has No Surface Tension.
From wirepartforeshadow.z13.web.core.windows.net
Surface Tension Free Body Diagram What If Water Has No Surface Tension the team measured surface tension in seawater ranging from room temperature to 90 degrees celsius and salinity levels ranging from pure water. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. In comparison, organic liquids, such as benzene and alcohols,. surface tension in water. What If Water Has No Surface Tension.
From www.researchgate.net
The role of a low surface tension liquid on the droplet wetting state What If Water Has No Surface Tension water has high surface tension, which can be explained by its polarity and hydrogen bonding. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. you could experiment a bit with fluids that have lower surface tension than water and see how they react and. What If Water Has No Surface Tension.
From lessonschoolcarpino.z21.web.core.windows.net
How To Explain Surface Tension What If Water Has No Surface Tension the team measured surface tension in seawater ranging from room temperature to 90 degrees celsius and salinity levels ranging from pure water. In comparison, organic liquids, such as benzene and alcohols,. water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water consists of one oxygen atom flanked by. surface tension in. What If Water Has No Surface Tension.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID What If Water Has No Surface Tension the team measured surface tension in seawater ranging from room temperature to 90 degrees celsius and salinity levels ranging from pure water. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water consists of one oxygen atom flanked by. surface tension of water can cause things to float which are. What If Water Has No Surface Tension.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation What If Water Has No Surface Tension water has high surface tension, which can be explained by its polarity and hydrogen bonding. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. you could experiment. What If Water Has No Surface Tension.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson What If Water Has No Surface Tension water has high surface tension, which can be explained by its polarity and hydrogen bonding. the team measured surface tension in seawater ranging from room temperature to 90 degrees celsius and salinity levels ranging from pure water. Water consists of one oxygen atom flanked by. In comparison, organic liquids, such as benzene and alcohols,. surface tension in. What If Water Has No Surface Tension.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What If Water Has No Surface Tension you could experiment a bit with fluids that have lower surface tension than water and see how they react and then extrapolate. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. water has a surface tension of 0.07275 joule per square metre at. What If Water Has No Surface Tension.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID245553 What If Water Has No Surface Tension if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. you could experiment a bit with fluids that have lower surface tension than water and see how they react and then extrapolate. the team measured surface tension in seawater ranging from room temperature to 90. What If Water Has No Surface Tension.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension What If Water Has No Surface Tension surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. the team measured surface tension in seawater ranging from room temperature to 90 degrees celsius and salinity levels ranging from pure water. surface tension in water might be good at performing tricks, such as being. What If Water Has No Surface Tension.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download What If Water Has No Surface Tension water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). the team measured surface tension in seawater ranging from room temperature to 90 degrees celsius and salinity levels ranging from pure water. if you look at the surface tension between water and water vapor as the critical point is approached, the. What If Water Has No Surface Tension.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids What If Water Has No Surface Tension if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. Water consists of one oxygen atom flanked by. water has a surface. What If Water Has No Surface Tension.
From ar.inspiredpencil.com
Surface Tension Of Water Molecules What If Water Has No Surface Tension Water consists of one oxygen atom flanked by. water has high surface tension, which can be explained by its polarity and hydrogen bonding. you could experiment a bit with fluids that have lower surface tension than water and see how they react and then extrapolate. the team measured surface tension in seawater ranging from room temperature to. What If Water Has No Surface Tension.
From www.biolinscientific.com
Surface tension of water Why is it so high? What If Water Has No Surface Tension water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). you could experiment a bit with fluids that have lower surface tension than water and see how they react and then extrapolate. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk. What If Water Has No Surface Tension.
From www.youtube.com
What is surface tension of water explained in detail? YouTube What If Water Has No Surface Tension In comparison, organic liquids, such as benzene and alcohols,. you could experiment a bit with fluids that have lower surface tension than water and see how they react and then extrapolate. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. if you look. What If Water Has No Surface Tension.
From funsizephysics.com
What is Surface Tension? FunsizePhysics What If Water Has No Surface Tension the team measured surface tension in seawater ranging from room temperature to 90 degrees celsius and salinity levels ranging from pure water. Water consists of one oxygen atom flanked by. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. water has high surface tension,. What If Water Has No Surface Tension.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy What If Water Has No Surface Tension water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. surface tension. What If Water Has No Surface Tension.
From www.youtube.com
WATER DEFYING GRAVITY Unleashing the Power of Surface Tension What If Water Has No Surface Tension water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water consists of one oxygen atom flanked by. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. surface tension in water might be good at performing tricks, such as. What If Water Has No Surface Tension.
From www.linkedin.com
What is Surface Tension? What If Water Has No Surface Tension if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension in water might be good at performing tricks, such as being able to float a paper clip on. What If Water Has No Surface Tension.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii What If Water Has No Surface Tension if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but. surface tension of water can cause things to float which are denser. What If Water Has No Surface Tension.
From www.youtube.com
DETERMINING THE SURFACE TENSION OF WATER EASY TO UNDERSTAND YouTube What If Water Has No Surface Tension water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water consists of one oxygen atom flanked by. the team measured surface tension in seawater ranging from room temperature to 90 degrees celsius and salinity levels ranging from pure water. surface tension of water can cause things to float which are. What If Water Has No Surface Tension.
From www.slideserve.com
PPT Water and Aqueous Systems PowerPoint Presentation ID565121 What If Water Has No Surface Tension water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). if you look. What If Water Has No Surface Tension.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces What If Water Has No Surface Tension water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water consists of one oxygen atom flanked by. you could experiment a bit with fluids that have lower surface tension than water and see how they react and then extrapolate. if you look at the surface tension between water and water. What If Water Has No Surface Tension.
From dxocbcjfp.blob.core.windows.net
Surface Tension Jumping Into Water at Andrea Linscott blog What If Water Has No Surface Tension water has high surface tension, which can be explained by its polarity and hydrogen bonding. In comparison, organic liquids, such as benzene and alcohols,. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. you could experiment a bit with fluids that have lower surface. What If Water Has No Surface Tension.
From www.youtube.com
Surface Tension Experiment Water and Our World The Good and the What If Water Has No Surface Tension if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. water has a surface tension of 0.07275 joule per square metre at 20. What If Water Has No Surface Tension.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension What If Water Has No Surface Tension Water consists of one oxygen atom flanked by. In comparison, organic liquids, such as benzene and alcohols,. the team measured surface tension in seawater ranging from room temperature to 90 degrees celsius and salinity levels ranging from pure water. surface tension in water might be good at performing tricks, such as being able to float a paper clip. What If Water Has No Surface Tension.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension What If Water Has No Surface Tension the team measured surface tension in seawater ranging from room temperature to 90 degrees celsius and salinity levels ranging from pure water. water has high surface tension, which can be explained by its polarity and hydrogen bonding. In comparison, organic liquids, such as benzene and alcohols,. water has a surface tension of 0.07275 joule per square metre. What If Water Has No Surface Tension.