Medical Device Regulations Taiwan . regulations governing contract manufacturing of medical devices. 2024 apec medical devices regulatory science center of excellence (coe) workshop; the medical devices act will establish a system to effectively regulate medical devices throughout the medical. regulations on good clinical practice for medical devices. in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. latest status of the medical devices regulation in taiwan. This act is established to ensure the safety, effectiveness, and quality of medical. Data source: food and drug administration, ministry of. medical devices are classified into the following categories according to their function, intended use, operating.
from www.youtube.com
2024 apec medical devices regulatory science center of excellence (coe) workshop; regulations on good clinical practice for medical devices. latest status of the medical devices regulation in taiwan. in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. regulations governing contract manufacturing of medical devices. This act is established to ensure the safety, effectiveness, and quality of medical. medical devices are classified into the following categories according to their function, intended use, operating. Data source: food and drug administration, ministry of. the medical devices act will establish a system to effectively regulate medical devices throughout the medical.
Global Medical Device Registration Impact of Changes to the EU MDR and
Medical Device Regulations Taiwan regulations on good clinical practice for medical devices. the medical devices act will establish a system to effectively regulate medical devices throughout the medical. in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. regulations governing contract manufacturing of medical devices. regulations on good clinical practice for medical devices. 2024 apec medical devices regulatory science center of excellence (coe) workshop; Data source: food and drug administration, ministry of. This act is established to ensure the safety, effectiveness, and quality of medical. medical devices are classified into the following categories according to their function, intended use, operating. latest status of the medical devices regulation in taiwan.
From www.slideshare.net
Taiwan medical device registration and approval chart EMERGO Medical Device Regulations Taiwan in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. Data source: food and drug administration, ministry of. regulations on good clinical practice for medical devices. regulations governing contract manufacturing of medical devices. latest status of the medical devices regulation in taiwan. medical devices are classified into the following categories. Medical Device Regulations Taiwan.
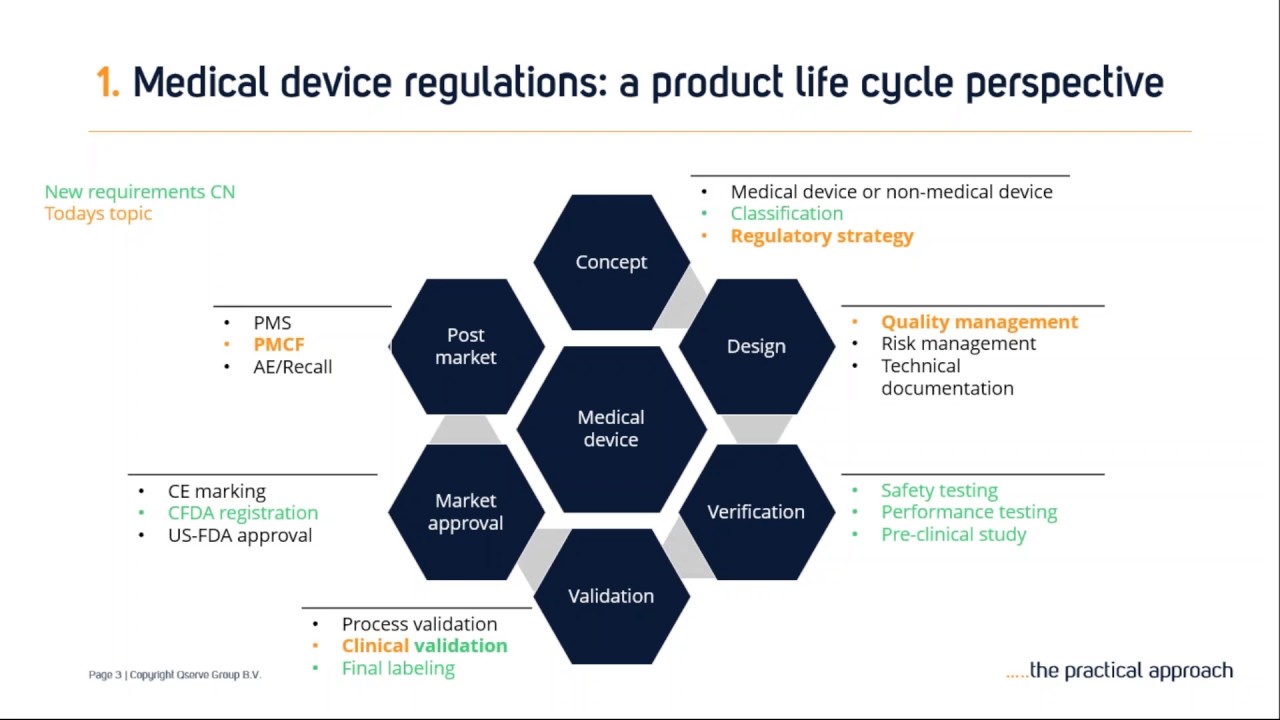
From www.scilife.io
The 5 Medical Device Development Phases Scilife Medical Device Regulations Taiwan the medical devices act will establish a system to effectively regulate medical devices throughout the medical. latest status of the medical devices regulation in taiwan. Data source: food and drug administration, ministry of. This act is established to ensure the safety, effectiveness, and quality of medical. medical devices are classified into the following categories according to their. Medical Device Regulations Taiwan.
From www.regdesk.co
Recent Changes to Medical Device Regulations in Canada RegDesk Medical Device Regulations Taiwan regulations governing contract manufacturing of medical devices. This act is established to ensure the safety, effectiveness, and quality of medical. Data source: food and drug administration, ministry of. regulations on good clinical practice for medical devices. in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. medical devices are classified into. Medical Device Regulations Taiwan.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Device Regulations Taiwan the medical devices act will establish a system to effectively regulate medical devices throughout the medical. in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. regulations on good clinical practice for medical devices. medical devices are classified into the following categories according to their function, intended use, operating. latest. Medical Device Regulations Taiwan.
From www.transcustoms.com
Medical Device NMPA Registration Procedrue Flow Chart Medical Device Regulations Taiwan This act is established to ensure the safety, effectiveness, and quality of medical. the medical devices act will establish a system to effectively regulate medical devices throughout the medical. medical devices are classified into the following categories according to their function, intended use, operating. latest status of the medical devices regulation in taiwan. Data source: food and. Medical Device Regulations Taiwan.
From www.slideshare.net
Global medical device regulations Medical Device Regulations Taiwan the medical devices act will establish a system to effectively regulate medical devices throughout the medical. This act is established to ensure the safety, effectiveness, and quality of medical. regulations governing contract manufacturing of medical devices. medical devices are classified into the following categories according to their function, intended use, operating. Data source: food and drug administration,. Medical Device Regulations Taiwan.
From www.pathologyinpractice.com
Implementation of future Medical Device Regulations delayed by 12 months Medical Device Regulations Taiwan latest status of the medical devices regulation in taiwan. medical devices are classified into the following categories according to their function, intended use, operating. 2024 apec medical devices regulatory science center of excellence (coe) workshop; Data source: food and drug administration, ministry of. regulations governing contract manufacturing of medical devices. in this part, standards related. Medical Device Regulations Taiwan.
From asiaactual.com
Thailand Implements New Medical Device Regulations Asia Actual, LLC Medical Device Regulations Taiwan This act is established to ensure the safety, effectiveness, and quality of medical. in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. medical devices are classified into the following categories according to their function, intended use, operating. latest status of the medical devices regulation in taiwan. regulations governing contract manufacturing. Medical Device Regulations Taiwan.
From crfweb.com
Medical Device Regulations Medical Device Regulations Taiwan the medical devices act will establish a system to effectively regulate medical devices throughout the medical. regulations on good clinical practice for medical devices. 2024 apec medical devices regulatory science center of excellence (coe) workshop; in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. This act is established to ensure. Medical Device Regulations Taiwan.
From www.konect.or.kr
KONECT 국가임상시험지원재단 Medical Device Regulations Taiwan the medical devices act will establish a system to effectively regulate medical devices throughout the medical. in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. latest status of the medical devices regulation in taiwan. medical devices are classified into the following categories according to their function, intended use, operating. This. Medical Device Regulations Taiwan.
From www.lek.com
European Medical Devices Regulations and Their Impact Medical Device Regulations Taiwan the medical devices act will establish a system to effectively regulate medical devices throughout the medical. regulations on good clinical practice for medical devices. Data source: food and drug administration, ministry of. latest status of the medical devices regulation in taiwan. medical devices are classified into the following categories according to their function, intended use, operating.. Medical Device Regulations Taiwan.
From www.cell.com
The Regulation of Wearable Medical Devices Trends in Biotechnology Medical Device Regulations Taiwan the medical devices act will establish a system to effectively regulate medical devices throughout the medical. regulations on good clinical practice for medical devices. Data source: food and drug administration, ministry of. regulations governing contract manufacturing of medical devices. medical devices are classified into the following categories according to their function, intended use, operating. This act. Medical Device Regulations Taiwan.
From www.researchsolutions.com
European Medical Device Regulation Guide to simplify compliance 2021 Medical Device Regulations Taiwan This act is established to ensure the safety, effectiveness, and quality of medical. medical devices are classified into the following categories according to their function, intended use, operating. Data source: food and drug administration, ministry of. regulations governing contract manufacturing of medical devices. in this part, standards related to the facilities, equipment, organization and personnel, production, quality. Medical Device Regulations Taiwan.
From www.apcerls.com
US Medical Device Regulations APCER Life Sciences Medical Device Regulations Taiwan latest status of the medical devices regulation in taiwan. in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. 2024 apec medical devices regulatory science center of excellence (coe) workshop; regulations governing contract manufacturing of medical devices. Data source: food and drug administration, ministry of. This act is established to ensure. Medical Device Regulations Taiwan.
From www.youtube.com
Understanding Medical Device Regulations YouTube Medical Device Regulations Taiwan 2024 apec medical devices regulatory science center of excellence (coe) workshop; the medical devices act will establish a system to effectively regulate medical devices throughout the medical. latest status of the medical devices regulation in taiwan. in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. This act is established to. Medical Device Regulations Taiwan.
From www.regdesk.co
Changes to the Swiss Medical Device Regulation RegDesk Medical Device Regulations Taiwan Data source: food and drug administration, ministry of. the medical devices act will establish a system to effectively regulate medical devices throughout the medical. medical devices are classified into the following categories according to their function, intended use, operating. regulations governing contract manufacturing of medical devices. latest status of the medical devices regulation in taiwan. . Medical Device Regulations Taiwan.
From www.emergobyul.com
TFDA Releases Details About Transition to New Taiwanese Medical Device Medical Device Regulations Taiwan This act is established to ensure the safety, effectiveness, and quality of medical. 2024 apec medical devices regulatory science center of excellence (coe) workshop; Data source: food and drug administration, ministry of. the medical devices act will establish a system to effectively regulate medical devices throughout the medical. in this part, standards related to the facilities, equipment,. Medical Device Regulations Taiwan.
From www.youtube.com
Global Medical Device Registration Impact of Changes to the EU MDR and Medical Device Regulations Taiwan medical devices are classified into the following categories according to their function, intended use, operating. 2024 apec medical devices regulatory science center of excellence (coe) workshop; Data source: food and drug administration, ministry of. the medical devices act will establish a system to effectively regulate medical devices throughout the medical. latest status of the medical devices. Medical Device Regulations Taiwan.
From www.tramremisedehallen.nl
Understanding Medical Devices Regulations to Guarantee Compliance Medical Device Regulations Taiwan Data source: food and drug administration, ministry of. regulations governing contract manufacturing of medical devices. latest status of the medical devices regulation in taiwan. regulations on good clinical practice for medical devices. This act is established to ensure the safety, effectiveness, and quality of medical. the medical devices act will establish a system to effectively regulate. Medical Device Regulations Taiwan.
From www.argosmultilingual.com
The New EU Medical Device Regulations Are You Prepared? Medical Device Regulations Taiwan medical devices are classified into the following categories according to their function, intended use, operating. in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. Data source: food and drug administration, ministry of. latest status of the medical devices regulation in taiwan. regulations governing contract manufacturing of medical devices. 2024. Medical Device Regulations Taiwan.
From medicaldialogues.in
Medical device industry needs flexibility in labour laws, tax Medical Device Regulations Taiwan medical devices are classified into the following categories according to their function, intended use, operating. in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. regulations on good clinical practice for medical devices. This act is established to ensure the safety, effectiveness, and quality of medical. Data source: food and drug administration,. Medical Device Regulations Taiwan.
From www.slideshare.net
Malaysia Medical Devices Regulations Medical Device Regulations Taiwan the medical devices act will establish a system to effectively regulate medical devices throughout the medical. in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. latest status of the medical devices regulation in taiwan. 2024 apec medical devices regulatory science center of excellence (coe) workshop; regulations governing contract manufacturing. Medical Device Regulations Taiwan.
From sterlingmedicaldevices.com
New Proposed FDA Medical Device Quality Control Regulations Sterling Medical Device Regulations Taiwan regulations on good clinical practice for medical devices. Data source: food and drug administration, ministry of. 2024 apec medical devices regulatory science center of excellence (coe) workshop; medical devices are classified into the following categories according to their function, intended use, operating. latest status of the medical devices regulation in taiwan. regulations governing contract manufacturing. Medical Device Regulations Taiwan.
From www.dogtownmedia.com
FDA Medical Device Regulations — Part 1 Dogtown Media Medical Device Regulations Taiwan medical devices are classified into the following categories according to their function, intended use, operating. regulations on good clinical practice for medical devices. latest status of the medical devices regulation in taiwan. This act is established to ensure the safety, effectiveness, and quality of medical. Data source: food and drug administration, ministry of. 2024 apec medical. Medical Device Regulations Taiwan.
From premier-research.com
Medical Device Trials What You Need to Know About U.S. Regulations Medical Device Regulations Taiwan regulations governing contract manufacturing of medical devices. the medical devices act will establish a system to effectively regulate medical devices throughout the medical. 2024 apec medical devices regulatory science center of excellence (coe) workshop; This act is established to ensure the safety, effectiveness, and quality of medical. Data source: food and drug administration, ministry of. in. Medical Device Regulations Taiwan.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Regulations Taiwan latest status of the medical devices regulation in taiwan. the medical devices act will establish a system to effectively regulate medical devices throughout the medical. 2024 apec medical devices regulatory science center of excellence (coe) workshop; This act is established to ensure the safety, effectiveness, and quality of medical. medical devices are classified into the following. Medical Device Regulations Taiwan.
From www.apcerls.com
EU Medical Device Regulations APCER Life Sciences Medical Device Regulations Taiwan in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. 2024 apec medical devices regulatory science center of excellence (coe) workshop; latest status of the medical devices regulation in taiwan. regulations on good clinical practice for medical devices. the medical devices act will establish a system to effectively regulate medical. Medical Device Regulations Taiwan.
From apacmed.org
Medical Device Regulation Importance and Examples in APAC Medical Device Regulations Taiwan Data source: food and drug administration, ministry of. 2024 apec medical devices regulatory science center of excellence (coe) workshop; medical devices are classified into the following categories according to their function, intended use, operating. This act is established to ensure the safety, effectiveness, and quality of medical. latest status of the medical devices regulation in taiwan. . Medical Device Regulations Taiwan.
From www.europeanpharmaceuticalreview.com
EU’s Medical Device Regulation is now applicable Medical Device Regulations Taiwan the medical devices act will establish a system to effectively regulate medical devices throughout the medical. This act is established to ensure the safety, effectiveness, and quality of medical. Data source: food and drug administration, ministry of. regulations on good clinical practice for medical devices. latest status of the medical devices regulation in taiwan. 2024 apec. Medical Device Regulations Taiwan.
From onedayatatimeinomaha.blogspot.com
Fda Class 2 Medical Device List Medical Blog Medical Device Regulations Taiwan in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. Data source: food and drug administration, ministry of. medical devices are classified into the following categories according to their function, intended use, operating. the medical devices act will establish a system to effectively regulate medical devices throughout the medical. regulations on. Medical Device Regulations Taiwan.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Medical Device Regulations Taiwan This act is established to ensure the safety, effectiveness, and quality of medical. regulations governing contract manufacturing of medical devices. Data source: food and drug administration, ministry of. in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. regulations on good clinical practice for medical devices. latest status of the medical. Medical Device Regulations Taiwan.
From www.bmedicalsystems.com
FAQ on the European Medical Device Regulation B Medical Systems (US) Medical Device Regulations Taiwan This act is established to ensure the safety, effectiveness, and quality of medical. regulations on good clinical practice for medical devices. medical devices are classified into the following categories according to their function, intended use, operating. Data source: food and drug administration, ministry of. the medical devices act will establish a system to effectively regulate medical devices. Medical Device Regulations Taiwan.
From abhipedia.abhimanu.com
Issues and Analysis on Medical Device Regulations In India for UPSC Medical Device Regulations Taiwan latest status of the medical devices regulation in taiwan. Data source: food and drug administration, ministry of. in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. 2024 apec medical devices regulatory science center of excellence (coe) workshop; regulations on good clinical practice for medical devices. medical devices are classified. Medical Device Regulations Taiwan.
From acmotorsandfabs.co.uk
Velo Reproducir Molesto medical equipment regulations período explorar Medical Device Regulations Taiwan Data source: food and drug administration, ministry of. in this part, standards related to the facilities, equipment, organization and personnel, production, quality control,. regulations governing contract manufacturing of medical devices. the medical devices act will establish a system to effectively regulate medical devices throughout the medical. This act is established to ensure the safety, effectiveness, and quality. Medical Device Regulations Taiwan.
From www.tuvsud.com
Infographic The New Medical Device Regulation TÜV SÜD in India Medical Device Regulations Taiwan Data source: food and drug administration, ministry of. medical devices are classified into the following categories according to their function, intended use, operating. regulations governing contract manufacturing of medical devices. the medical devices act will establish a system to effectively regulate medical devices throughout the medical. latest status of the medical devices regulation in taiwan. . Medical Device Regulations Taiwan.