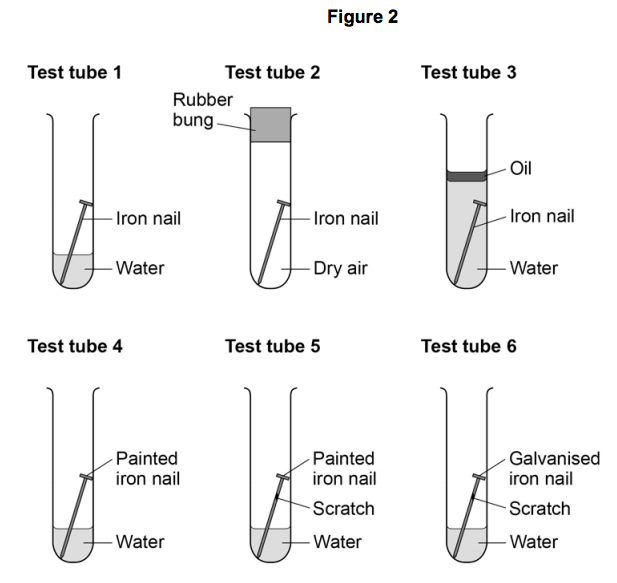
How Long Does It Take An Iron Nail Exposed To The Rain To Rust . fresh iron exposed to a hot atmosphere with plenty of oxygen and water will form a thin layer of rust immediately (although if you. In this chemistry science fair project, you'll investigate how acids change the rate of rusting as you compare a model of rainwater and models for acid rain. The soda and juices should not cause any rust to form on the nail. The acceleration of the corrosion procedure can be observed when the value of the ph of the metal’s immediate surroundings is low. What if you use iron wool and iron filings instead? observe the nails daily to check for rust formation. revision notes on rusting of iron for the cambridge o level chemistry syllabus, written by the chemistry experts at save. the iron nail in test tube e will begin to rust first, followed by the iron nails in test tubes c, b, and a. The rate of iron corrosion is accelerated upon exposure to acid rain. the primary cause of corrosion is being exposed to precipitation, particularly rain. with a thermometer and a timer, you can measure how fast heat is being given off (the rate), and that will give you an idea of how fast the reaction is occurring. rusting of iron refers to the formation of rust, a mixture of iron oxides, on the surface of iron objects or structures. The nails in water should both form rust within three weeks, and the vinegar should rust a nail approximately one week later. if you scratch the nail first, will it rust faster or slower? The iron nail in test tube d should be the last.
from ar.inspiredpencil.com
The nails in water should both form rust within three weeks, and the vinegar should rust a nail approximately one week later. if you scratch the nail first, will it rust faster or slower? The soda and juices should not cause any rust to form on the nail. In this chemistry science fair project, you'll investigate how acids change the rate of rusting as you compare a model of rainwater and models for acid rain. The rate of iron corrosion is accelerated upon exposure to acid rain. The iron nail in test tube d should be the last. rusting of iron refers to the formation of rust, a mixture of iron oxides, on the surface of iron objects or structures. the primary cause of corrosion is being exposed to precipitation, particularly rain. with a thermometer and a timer, you can measure how fast heat is being given off (the rate), and that will give you an idea of how fast the reaction is occurring. the iron nail in test tube e will begin to rust first, followed by the iron nails in test tubes c, b, and a.
Rusting Of Iron Nails
How Long Does It Take An Iron Nail Exposed To The Rain To Rust The rate of iron corrosion is accelerated upon exposure to acid rain. The soda and juices should not cause any rust to form on the nail. The iron nail in test tube d should be the last. The acceleration of the corrosion procedure can be observed when the value of the ph of the metal’s immediate surroundings is low. The rate of iron corrosion is accelerated upon exposure to acid rain. fresh iron exposed to a hot atmosphere with plenty of oxygen and water will form a thin layer of rust immediately (although if you. rusting of iron refers to the formation of rust, a mixture of iron oxides, on the surface of iron objects or structures. observe the nails daily to check for rust formation. The nails in water should both form rust within three weeks, and the vinegar should rust a nail approximately one week later. What if you use iron wool and iron filings instead? with a thermometer and a timer, you can measure how fast heat is being given off (the rate), and that will give you an idea of how fast the reaction is occurring. the iron nail in test tube e will begin to rust first, followed by the iron nails in test tubes c, b, and a. In this chemistry science fair project, you'll investigate how acids change the rate of rusting as you compare a model of rainwater and models for acid rain. the primary cause of corrosion is being exposed to precipitation, particularly rain. if you scratch the nail first, will it rust faster or slower? revision notes on rusting of iron for the cambridge o level chemistry syllabus, written by the chemistry experts at save.
From www.fizzicseducation.com.au
Rusty nail experiment Fizzics Education How Long Does It Take An Iron Nail Exposed To The Rain To Rust The acceleration of the corrosion procedure can be observed when the value of the ph of the metal’s immediate surroundings is low. the iron nail in test tube e will begin to rust first, followed by the iron nails in test tubes c, b, and a. if you scratch the nail first, will it rust faster or slower?. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From wd40.africa
Guide on How to Clean Rust from Your Wrought Iron Furniture How Long Does It Take An Iron Nail Exposed To The Rain To Rust the iron nail in test tube e will begin to rust first, followed by the iron nails in test tubes c, b, and a. What if you use iron wool and iron filings instead? with a thermometer and a timer, you can measure how fast heat is being given off (the rate), and that will give you an. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From mybios.me
Rusting Iron Nails Experiment Bios Pics How Long Does It Take An Iron Nail Exposed To The Rain To Rust The rate of iron corrosion is accelerated upon exposure to acid rain. the iron nail in test tube e will begin to rust first, followed by the iron nails in test tubes c, b, and a. observe the nails daily to check for rust formation. with a thermometer and a timer, you can measure how fast heat. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From www.freepik.com
Premium Photo Rusty nail Many rusted nails Group of Iron rust Metal How Long Does It Take An Iron Nail Exposed To The Rain To Rust rusting of iron refers to the formation of rust, a mixture of iron oxides, on the surface of iron objects or structures. if you scratch the nail first, will it rust faster or slower? the primary cause of corrosion is being exposed to precipitation, particularly rain. The nails in water should both form rust within three weeks,. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From joelgordon.photoshelter.com
Iron Nails rate of Rusting chemisty experiement Joel Gordon Photography How Long Does It Take An Iron Nail Exposed To The Rain To Rust the primary cause of corrosion is being exposed to precipitation, particularly rain. with a thermometer and a timer, you can measure how fast heat is being given off (the rate), and that will give you an idea of how fast the reaction is occurring. What if you use iron wool and iron filings instead? if you scratch. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From www.dreamstime.com
Rusty Iron Boards Exposed To Heat and Rain Stock Image Image of rain How Long Does It Take An Iron Nail Exposed To The Rain To Rust the primary cause of corrosion is being exposed to precipitation, particularly rain. The rate of iron corrosion is accelerated upon exposure to acid rain. fresh iron exposed to a hot atmosphere with plenty of oxygen and water will form a thin layer of rust immediately (although if you. revision notes on rusting of iron for the cambridge. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From www.dreamstime.com
Many Rusted Nail,Group of Iron Rust,Metal Surface Brown from How Long Does It Take An Iron Nail Exposed To The Rain To Rust What if you use iron wool and iron filings instead? observe the nails daily to check for rust formation. The soda and juices should not cause any rust to form on the nail. In this chemistry science fair project, you'll investigate how acids change the rate of rusting as you compare a model of rainwater and models for acid. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From ceg.edu.vn
Discover 148+ pure iron nails super hot ceg.edu.vn How Long Does It Take An Iron Nail Exposed To The Rain To Rust The rate of iron corrosion is accelerated upon exposure to acid rain. What if you use iron wool and iron filings instead? The acceleration of the corrosion procedure can be observed when the value of the ph of the metal’s immediate surroundings is low. observe the nails daily to check for rust formation. the iron nail in test. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From matehope54.pythonanywhere.com
First Class Info About How To Prevent Rusting On Iron Nails Matehope54 How Long Does It Take An Iron Nail Exposed To The Rain To Rust rusting of iron refers to the formation of rust, a mixture of iron oxides, on the surface of iron objects or structures. The iron nail in test tube d should be the last. fresh iron exposed to a hot atmosphere with plenty of oxygen and water will form a thin layer of rust immediately (although if you. . How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From ar.inspiredpencil.com
Rusting Of Iron Diagram How Long Does It Take An Iron Nail Exposed To The Rain To Rust observe the nails daily to check for rust formation. fresh iron exposed to a hot atmosphere with plenty of oxygen and water will form a thin layer of rust immediately (although if you. The iron nail in test tube d should be the last. In this chemistry science fair project, you'll investigate how acids change the rate of. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From flatdisk24.pythonanywhere.com
How To Prevent An Iron Nail From Rusting Flatdisk24 How Long Does It Take An Iron Nail Exposed To The Rain To Rust What if you use iron wool and iron filings instead? The soda and juices should not cause any rust to form on the nail. observe the nails daily to check for rust formation. The nails in water should both form rust within three weeks, and the vinegar should rust a nail approximately one week later. In this chemistry science. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From animalia-life.club
Rusted Iron Nail How Long Does It Take An Iron Nail Exposed To The Rain To Rust if you scratch the nail first, will it rust faster or slower? The acceleration of the corrosion procedure can be observed when the value of the ph of the metal’s immediate surroundings is low. observe the nails daily to check for rust formation. The rate of iron corrosion is accelerated upon exposure to acid rain. The nails in. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From www.dreamstime.com
Rusty Iron Nails stock photo. Image of antique, grunge 91775590 How Long Does It Take An Iron Nail Exposed To The Rain To Rust The rate of iron corrosion is accelerated upon exposure to acid rain. the primary cause of corrosion is being exposed to precipitation, particularly rain. observe the nails daily to check for rust formation. revision notes on rusting of iron for the cambridge o level chemistry syllabus, written by the chemistry experts at save. The iron nail in. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From flatdisk24.pythonanywhere.com
How To Prevent An Iron Nail From Rusting Flatdisk24 How Long Does It Take An Iron Nail Exposed To The Rain To Rust The soda and juices should not cause any rust to form on the nail. In this chemistry science fair project, you'll investigate how acids change the rate of rusting as you compare a model of rainwater and models for acid rain. rusting of iron refers to the formation of rust, a mixture of iron oxides, on the surface of. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From www.indiamart.com
2inch Galvanized Iron Nail, 5 Gauge at Rs 70/kg in Gurugram ID How Long Does It Take An Iron Nail Exposed To The Rain To Rust The nails in water should both form rust within three weeks, and the vinegar should rust a nail approximately one week later. the primary cause of corrosion is being exposed to precipitation, particularly rain. The acceleration of the corrosion procedure can be observed when the value of the ph of the metal’s immediate surroundings is low. if you. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From www.picxy.com
Image of Iron Rusted NailsRK792852Picxy How Long Does It Take An Iron Nail Exposed To The Rain To Rust the iron nail in test tube e will begin to rust first, followed by the iron nails in test tubes c, b, and a. In this chemistry science fair project, you'll investigate how acids change the rate of rusting as you compare a model of rainwater and models for acid rain. with a thermometer and a timer, you. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From www.alamy.com
Rusting of iron nail experiment diagram. Investigating the conditions How Long Does It Take An Iron Nail Exposed To The Rain To Rust What if you use iron wool and iron filings instead? The acceleration of the corrosion procedure can be observed when the value of the ph of the metal’s immediate surroundings is low. if you scratch the nail first, will it rust faster or slower? fresh iron exposed to a hot atmosphere with plenty of oxygen and water will. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From ar.inspiredpencil.com
Rusting Of Iron Nails How Long Does It Take An Iron Nail Exposed To The Rain To Rust rusting of iron refers to the formation of rust, a mixture of iron oxides, on the surface of iron objects or structures. observe the nails daily to check for rust formation. In this chemistry science fair project, you'll investigate how acids change the rate of rusting as you compare a model of rainwater and models for acid rain.. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From www.shutterstock.com
21,333 Rusted iron nail Stock Photos, Images & Photography Shutterstock How Long Does It Take An Iron Nail Exposed To The Rain To Rust if you scratch the nail first, will it rust faster or slower? In this chemistry science fair project, you'll investigate how acids change the rate of rusting as you compare a model of rainwater and models for acid rain. What if you use iron wool and iron filings instead? The iron nail in test tube d should be the. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From ar.inspiredpencil.com
Rusting Of Iron Nails How Long Does It Take An Iron Nail Exposed To The Rain To Rust rusting of iron refers to the formation of rust, a mixture of iron oxides, on the surface of iron objects or structures. The rate of iron corrosion is accelerated upon exposure to acid rain. if you scratch the nail first, will it rust faster or slower? observe the nails daily to check for rust formation. The acceleration. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From pikbest.com
A Long Iron Nail Tool Illustration PNG Images PSD Free Download Pikbest How Long Does It Take An Iron Nail Exposed To The Rain To Rust the iron nail in test tube e will begin to rust first, followed by the iron nails in test tubes c, b, and a. fresh iron exposed to a hot atmosphere with plenty of oxygen and water will form a thin layer of rust immediately (although if you. the primary cause of corrosion is being exposed to. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From sciencing.com
Experiments on the Rusting of Iron Nails Sciencing How Long Does It Take An Iron Nail Exposed To The Rain To Rust What if you use iron wool and iron filings instead? The soda and juices should not cause any rust to form on the nail. The acceleration of the corrosion procedure can be observed when the value of the ph of the metal’s immediate surroundings is low. with a thermometer and a timer, you can measure how fast heat is. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From bramhamprimary.co.uk
Does the liquid affect how the iron nail rusts? Bramham Primary School How Long Does It Take An Iron Nail Exposed To The Rain To Rust the iron nail in test tube e will begin to rust first, followed by the iron nails in test tubes c, b, and a. revision notes on rusting of iron for the cambridge o level chemistry syllabus, written by the chemistry experts at save. The nails in water should both form rust within three weeks, and the vinegar. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From www.sciencephoto.com
Nails Rusting (After) Stock Image C001/7461 Science Photo Library How Long Does It Take An Iron Nail Exposed To The Rain To Rust What if you use iron wool and iron filings instead? The soda and juices should not cause any rust to form on the nail. fresh iron exposed to a hot atmosphere with plenty of oxygen and water will form a thin layer of rust immediately (although if you. The nails in water should both form rust within three weeks,. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From www.dreamstime.com
Rusted Iron Nail stock photo. Image of architecture, dirty 12829530 How Long Does It Take An Iron Nail Exposed To The Rain To Rust In this chemistry science fair project, you'll investigate how acids change the rate of rusting as you compare a model of rainwater and models for acid rain. The acceleration of the corrosion procedure can be observed when the value of the ph of the metal’s immediate surroundings is low. if you scratch the nail first, will it rust faster. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From www.youtube.com
Nail Rust (Fast) YouTube How Long Does It Take An Iron Nail Exposed To The Rain To Rust revision notes on rusting of iron for the cambridge o level chemistry syllabus, written by the chemistry experts at save. fresh iron exposed to a hot atmosphere with plenty of oxygen and water will form a thin layer of rust immediately (although if you. the iron nail in test tube e will begin to rust first, followed. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From www.youtube.com
Reaction Copper Sulphate and iron nailChapter6Class7 Science How Long Does It Take An Iron Nail Exposed To The Rain To Rust the primary cause of corrosion is being exposed to precipitation, particularly rain. What if you use iron wool and iron filings instead? observe the nails daily to check for rust formation. The acceleration of the corrosion procedure can be observed when the value of the ph of the metal’s immediate surroundings is low. The soda and juices should. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From www.toppr.com
In which test tubes, the rusting of iron nail will take place? How Long Does It Take An Iron Nail Exposed To The Rain To Rust The soda and juices should not cause any rust to form on the nail. In this chemistry science fair project, you'll investigate how acids change the rate of rusting as you compare a model of rainwater and models for acid rain. The rate of iron corrosion is accelerated upon exposure to acid rain. with a thermometer and a timer,. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From shapeguidance1.gitlab.io
Fun Evidence Of Chemical Reaction Rusting An Iron Nail Vce Physics How Long Does It Take An Iron Nail Exposed To The Rain To Rust The rate of iron corrosion is accelerated upon exposure to acid rain. In this chemistry science fair project, you'll investigate how acids change the rate of rusting as you compare a model of rainwater and models for acid rain. revision notes on rusting of iron for the cambridge o level chemistry syllabus, written by the chemistry experts at save.. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From teachernotes4u.com
A student set up the following experiment to investigate the conditions How Long Does It Take An Iron Nail Exposed To The Rain To Rust observe the nails daily to check for rust formation. with a thermometer and a timer, you can measure how fast heat is being given off (the rate), and that will give you an idea of how fast the reaction is occurring. The acceleration of the corrosion procedure can be observed when the value of the ph of the. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From www.nagwa.com
Question Video Understanding What Happens to the Mass of an Iron Nail How Long Does It Take An Iron Nail Exposed To The Rain To Rust What if you use iron wool and iron filings instead? revision notes on rusting of iron for the cambridge o level chemistry syllabus, written by the chemistry experts at save. the primary cause of corrosion is being exposed to precipitation, particularly rain. the iron nail in test tube e will begin to rust first, followed by the. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From www.vecteezy.com
Rusting of iron nail experiment diagram. 27798472 Vector Art at Vecteezy How Long Does It Take An Iron Nail Exposed To The Rain To Rust with a thermometer and a timer, you can measure how fast heat is being given off (the rate), and that will give you an idea of how fast the reaction is occurring. In this chemistry science fair project, you'll investigate how acids change the rate of rusting as you compare a model of rainwater and models for acid rain.. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From ar.inspiredpencil.com
Rusting Of Iron Nails How Long Does It Take An Iron Nail Exposed To The Rain To Rust rusting of iron refers to the formation of rust, a mixture of iron oxides, on the surface of iron objects or structures. fresh iron exposed to a hot atmosphere with plenty of oxygen and water will form a thin layer of rust immediately (although if you. What if you use iron wool and iron filings instead? the. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From online-learning-college.com
Rusting of iron Why does Iron rust? Chemical equation How Long Does It Take An Iron Nail Exposed To The Rain To Rust fresh iron exposed to a hot atmosphere with plenty of oxygen and water will form a thin layer of rust immediately (although if you. The rate of iron corrosion is accelerated upon exposure to acid rain. The soda and juices should not cause any rust to form on the nail. with a thermometer and a timer, you can. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.
From learningmediaautotelic.z22.web.core.windows.net
Is A Nail Rusting A Chemical Reaction How Long Does It Take An Iron Nail Exposed To The Rain To Rust if you scratch the nail first, will it rust faster or slower? the primary cause of corrosion is being exposed to precipitation, particularly rain. revision notes on rusting of iron for the cambridge o level chemistry syllabus, written by the chemistry experts at save. The soda and juices should not cause any rust to form on the. How Long Does It Take An Iron Nail Exposed To The Rain To Rust.