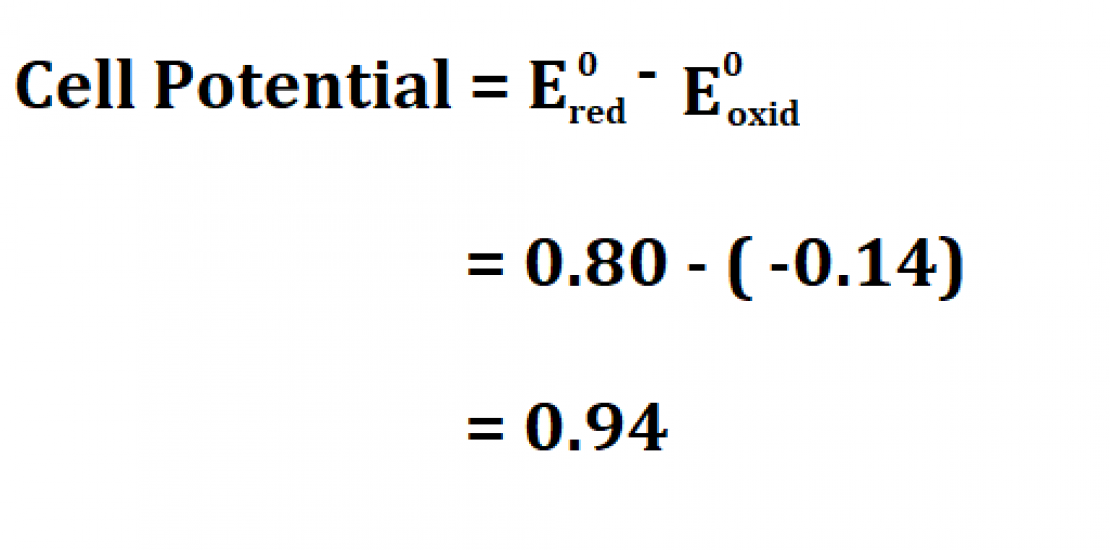Standard Cell Potential Formula . The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25 c) is called. Calculating the standard cell potential calculate the standard cell potential for the electrochemical cell below and explain why the cu 2+ / cu half. Interpret electrode potentials in terms of relative oxidant and reductant. To find the difference of the two half cells, the following. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. See examples of calculating cell potential for redox reactions. Describe and relate the definitions of electrode and cell potentials.
from www.learntocalculate.com
See examples of calculating cell potential for redox reactions. Interpret electrode potentials in terms of relative oxidant and reductant. Calculating the standard cell potential calculate the standard cell potential for the electrochemical cell below and explain why the cu 2+ / cu half. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. To find the difference of the two half cells, the following. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Describe and relate the definitions of electrode and cell potentials. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25 c) is called. Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells.
How to Calculate Cell Potential.
Standard Cell Potential Formula See examples of calculating cell potential for redox reactions. Calculating the standard cell potential calculate the standard cell potential for the electrochemical cell below and explain why the cu 2+ / cu half. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: To find the difference of the two half cells, the following. See examples of calculating cell potential for redox reactions. Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Interpret electrode potentials in terms of relative oxidant and reductant. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25 c) is called. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Describe and relate the definitions of electrode and cell potentials.
From www.youtube.com
2039 Calculating Standard Cell Potentials YouTube Standard Cell Potential Formula See examples of calculating cell potential for redox reactions. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials. Standard Cell Potential Formula.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID7041672 Standard Cell Potential Formula The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25 c) is called. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. See examples of calculating cell potential for redox reactions.. Standard Cell Potential Formula.
From www.slideserve.com
PPT Cell Potential and Nernst Equation PowerPoint Presentation, free Standard Cell Potential Formula The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25 c) is called. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. This is also known as the standard cell potential (e cell ). Standard Cell Potential Formula.
From www.chegg.com
Solved Using the Standard Cell Potential Equation, Ecell = Standard Cell Potential Formula Interpret electrode potentials in terms of relative oxidant and reductant. Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. Describe and relate the definitions of electrode and cell potentials. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. This is also known as the standard. Standard Cell Potential Formula.
From www.youtube.com
Calculating the Cell Potential of Electrochemical Cells. (Adv Chem Ch Standard Cell Potential Formula To find the difference of the two half cells, the following. Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. Calculating the standard cell potential calculate the standard cell potential for the electrochemical cell below and explain why the cu 2+ / cu half. See examples of calculating cell potential for redox. Standard Cell Potential Formula.
From www.slideserve.com
PPT Unit 09 Oxidation and Reduction PowerPoint Presentation, free Standard Cell Potential Formula The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25 c) is called. Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. The standard cell potential (\(e^o_{cell}\)) is the difference of the two. Standard Cell Potential Formula.
From www.youtube.com
The Nernst Equation and Concentration Cells Plus Examples YouTube Standard Cell Potential Formula The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Describe and relate the definitions of electrode and cell potentials. See examples of calculating cell potential for redox reactions. Interpret electrode potentials in terms of relative oxidant and reductant. Calculating the standard cell potential calculate the standard cell potential for the. Standard Cell Potential Formula.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Cell Potential Formula 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Calculating the standard cell potential calculate the standard cell potential for the electrochemical cell below and explain why the cu 2+ / cu half. To find the difference of the two half cells, the following. Learn the definition and formula of standard cell potential,. Standard Cell Potential Formula.
From studymarxianism.z21.web.core.windows.net
How To Calculate E Cell Potential Standard Cell Potential Formula Interpret electrode potentials in terms of relative oxidant and reductant. To find the difference of the two half cells, the following. See examples of calculating cell potential for redox reactions. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. This is also known as the standard cell potential (e cell. Standard Cell Potential Formula.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID321506 Standard Cell Potential Formula Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: Calculating the standard cell potential calculate the standard cell potential for the electrochemical cell below and explain why the cu 2+. Standard Cell Potential Formula.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential Formula See examples of calculating cell potential for redox reactions. Interpret electrode potentials in terms of relative oxidant and reductant. To find the difference of the two half cells, the following. Describe and relate the definitions of electrode and cell potentials. Calculating the standard cell potential calculate the standard cell potential for the electrochemical cell below and explain why the cu. Standard Cell Potential Formula.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Copper Standard Cell Potential Formula The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25 c) is called. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Interpret electrode potentials in terms of relative oxidant and. Standard Cell Potential Formula.
From www.youtube.com
calculation of half cell potential electrochemistry 2 class 12 Standard Cell Potential Formula This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: See examples of calculating cell potential for redox reactions. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The potential of the cell under standard conditions (1 m. Standard Cell Potential Formula.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential Formula The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: Calculating the standard cell potential calculate the standard cell potential for the electrochemical cell below and explain why the. Standard Cell Potential Formula.
From www.slideserve.com
PPT Calculating the Cell Potential PowerPoint Presentation, free Standard Cell Potential Formula Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for. Standard Cell Potential Formula.
From gaskatel.de
Principles of the pHvalue measurement with hydrogen electrodes Gaskatel Standard Cell Potential Formula Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: 42 rows calculate the standard cell potential of a. Standard Cell Potential Formula.
From scienceinfo.com
Cell Potential & Standard Cell Potential Standard Cell Potential Formula Interpret electrode potentials in terms of relative oxidant and reductant. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: To find the difference of the two half cells, the following. Describe. Standard Cell Potential Formula.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential Formula To find the difference of the two half cells, the following. Calculating the standard cell potential calculate the standard cell potential for the electrochemical cell below and explain why the cu 2+ / cu half. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: Describe and relate. Standard Cell Potential Formula.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps Standard Cell Potential Formula Describe and relate the definitions of electrode and cell potentials. Calculating the standard cell potential calculate the standard cell potential for the electrochemical cell below and explain why the cu 2+ / cu half. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a. Standard Cell Potential Formula.
From www.slideserve.com
PPT Nernst Equation PowerPoint Presentation, free download ID6600057 Standard Cell Potential Formula See examples of calculating cell potential for redox reactions. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. Calculating the standard cell potential calculate the standard cell potential for the electrochemical cell below. Standard Cell Potential Formula.
From www.chegg.com
Solved The Nernst Equation enables the determination of cell Standard Cell Potential Formula The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25 c) is called. Describe and relate the definitions of electrode and cell potentials. To find the difference of the two half cells, the following. 42 rows calculate the standard cell potential. Standard Cell Potential Formula.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential Formula This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. See examples. Standard Cell Potential Formula.
From salma-has-blevins.blogspot.com
How to Calculate Cell Potential Under Standard Conditions Salmahas Standard Cell Potential Formula Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. Calculating the standard cell potential calculate the standard cell potential for the electrochemical cell below and explain why the cu 2+ / cu half. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases,. Standard Cell Potential Formula.
From www.toppr.com
Calculate the potential half cell containing 0.10 M , K_2Cr_2 O_7 (aq Standard Cell Potential Formula The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. The potential of the cell under standard conditions. Standard Cell Potential Formula.
From www.youtube.com
Calculating Cell Potential (Ecell) under Nonstandard Conditions YouTube Standard Cell Potential Formula Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25 c) is called. Describe and relate the definitions of electrode and cell potentials. Interpret. Standard Cell Potential Formula.
From www.learntocalculate.com
How to Calculate Cell Potential. Standard Cell Potential Formula This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Describe and relate the definitions of electrode and cell. Standard Cell Potential Formula.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 Standard Cell Potential Formula Interpret electrode potentials in terms of relative oxidant and reductant. Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. This is also known as the standard cell potential (e cell ) the standard cell potential can. Standard Cell Potential Formula.
From www.tessshebaylo.com
What Is The Equation Used To Determine Electric Potential Energy Standard Cell Potential Formula Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. See examples of calculating cell potential for redox reactions. Describe and relate the definitions of electrode and cell potentials. The potential of the cell. Standard Cell Potential Formula.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Formula 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. Describe and relate the definitions of electrode and cell potentials. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of. Standard Cell Potential Formula.
From navaznajeeha.blogspot.com
34+ voltaic cell potential calculator NavazNajeeha Standard Cell Potential Formula Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. See examples of calculating cell potential for redox reactions. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms. Standard Cell Potential Formula.
From www.vrogue.co
What Is Cell Potential Definition Formula And Example vrogue.co Standard Cell Potential Formula Calculating the standard cell potential calculate the standard cell potential for the electrochemical cell below and explain why the cu 2+ / cu half. See examples of calculating cell potential for redox reactions. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: The standard cell potential (\(e^o_{cell}\)). Standard Cell Potential Formula.
From www.vrogue.co
What Is Cell Potential Definition Formula And Example vrogue.co Standard Cell Potential Formula 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The potential of the cell under standard conditions. Standard Cell Potential Formula.
From 2012books.lardbucket.org
Standard Potentials Standard Cell Potential Formula See examples of calculating cell potential for redox reactions. This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: Learn the definition and formula of standard cell potential, and how to apply it to electrochemical cells. Calculating the standard cell potential calculate the standard cell potential for the. Standard Cell Potential Formula.
From www.youtube.com
How to Calculate Standard Cell Potential and Voltage using E cell = E Standard Cell Potential Formula To find the difference of the two half cells, the following. Describe and relate the definitions of electrode and cell potentials. See examples of calculating cell potential for redox reactions. Calculating the standard cell potential calculate the standard cell potential for the electrochemical cell below and explain why the cu 2+ / cu half. Interpret electrode potentials in terms of. Standard Cell Potential Formula.
From www.nagwa.com
Question Video Calculating a Standard Cell Potential from Standard Standard Cell Potential Formula This is also known as the standard cell potential (e cell ) the standard cell potential can be determined by two methods: To find the difference of the two half cells, the following. Describe and relate the definitions of electrode and cell potentials. See examples of calculating cell potential for redox reactions. 42 rows calculate the standard cell potential of. Standard Cell Potential Formula.