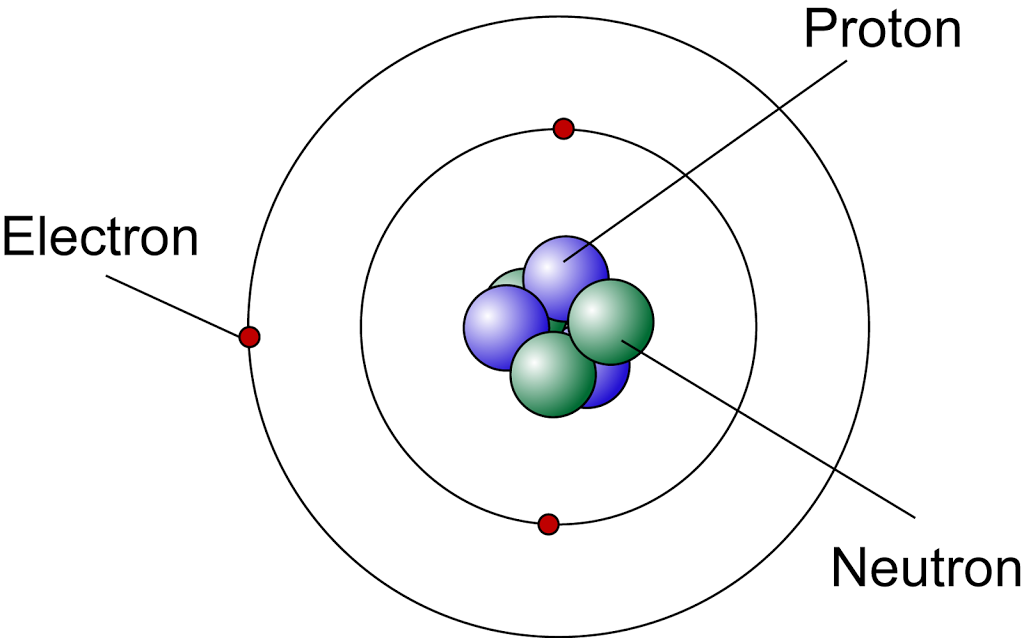
Iron Neutrons . Learn about the atomic number, mass number, and isotopes of atoms. The chemical symbol for iron is fe. Learn about the location, charge, and relative mass of neutrons, the subatomic particles that act as nuclear glue in the nucleus. Mass numbers of typical isotopes of iron are 56; The number of neutrons can be different, even in atoms of the same element. Learn about the number and arrangement of protons, neutrons, and electrons in iron, a common metal element with atomic number 26. Find out the stable and unstable isotopes of iron, and the most common alloy of iron, carbon steel. Atoms of the same element that contain the same number of. Find out how many protons, neutrons, and electrons are. Find out how to calculate the mass number. Neutron number and mass number of iron. Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26 protons and variable number of neutrons.
from
Find out the stable and unstable isotopes of iron, and the most common alloy of iron, carbon steel. Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26 protons and variable number of neutrons. Neutron number and mass number of iron. The chemical symbol for iron is fe. Atoms of the same element that contain the same number of. Mass numbers of typical isotopes of iron are 56; The number of neutrons can be different, even in atoms of the same element. Learn about the location, charge, and relative mass of neutrons, the subatomic particles that act as nuclear glue in the nucleus. Find out how to calculate the mass number. Learn about the atomic number, mass number, and isotopes of atoms.
Iron Neutrons The number of neutrons can be different, even in atoms of the same element. Mass numbers of typical isotopes of iron are 56; Neutron number and mass number of iron. Find out how many protons, neutrons, and electrons are. Learn about the location, charge, and relative mass of neutrons, the subatomic particles that act as nuclear glue in the nucleus. Learn about the atomic number, mass number, and isotopes of atoms. Atoms of the same element that contain the same number of. The number of neutrons can be different, even in atoms of the same element. Learn about the number and arrangement of protons, neutrons, and electrons in iron, a common metal element with atomic number 26. The chemical symbol for iron is fe. Find out the stable and unstable isotopes of iron, and the most common alloy of iron, carbon steel. Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26 protons and variable number of neutrons. Find out how to calculate the mass number.
From reviewhomedecor.co
Iron Periodic Table Protons Neutrons Electrons Review Home Decor Iron Neutrons Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26 protons and variable number of neutrons. Learn about the atomic number, mass number, and isotopes of atoms. The chemical symbol for iron is fe. Mass numbers of typical isotopes of iron are 56; Find out the stable and unstable isotopes of iron,. Iron Neutrons.
From
Iron Neutrons Find out how to calculate the mass number. Learn about the location, charge, and relative mass of neutrons, the subatomic particles that act as nuclear glue in the nucleus. The number of neutrons can be different, even in atoms of the same element. Learn about the atomic number, mass number, and isotopes of atoms. Atoms of the same element that. Iron Neutrons.
From
Iron Neutrons The number of neutrons can be different, even in atoms of the same element. Neutron number and mass number of iron. Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26 protons and variable number of neutrons. Learn about the atomic number, mass number, and isotopes of atoms. Find out how to. Iron Neutrons.
From
Iron Neutrons Find out the stable and unstable isotopes of iron, and the most common alloy of iron, carbon steel. Learn about the atomic number, mass number, and isotopes of atoms. Neutron number and mass number of iron. Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26 protons and variable number of neutrons.. Iron Neutrons.
From
Iron Neutrons Find out how to calculate the mass number. Learn about the atomic number, mass number, and isotopes of atoms. Learn about the location, charge, and relative mass of neutrons, the subatomic particles that act as nuclear glue in the nucleus. The chemical symbol for iron is fe. Atoms of the same element that contain the same number of. The number. Iron Neutrons.
From www.researchgate.net
The total crosssection of neutrons on iron target is shown versus the Iron Neutrons Find out the stable and unstable isotopes of iron, and the most common alloy of iron, carbon steel. Neutron number and mass number of iron. Atoms of the same element that contain the same number of. Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26 protons and variable number of neutrons.. Iron Neutrons.
From
Iron Neutrons Atoms of the same element that contain the same number of. Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26 protons and variable number of neutrons. Learn about the atomic number, mass number, and isotopes of atoms. Mass numbers of typical isotopes of iron are 56; Find out how many protons,. Iron Neutrons.
From
Iron Neutrons Learn about the location, charge, and relative mass of neutrons, the subatomic particles that act as nuclear glue in the nucleus. Learn about the atomic number, mass number, and isotopes of atoms. The number of neutrons can be different, even in atoms of the same element. Atoms of the same element that contain the same number of. Neutron number and. Iron Neutrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Iron Neutrons Neutron number and mass number of iron. Learn about the atomic number, mass number, and isotopes of atoms. Find out the stable and unstable isotopes of iron, and the most common alloy of iron, carbon steel. The number of neutrons can be different, even in atoms of the same element. Atoms of the same element that contain the same number. Iron Neutrons.
From reviewhomedecor.co
Iron Periodic Table Number Of Neutrons Review Home Decor Iron Neutrons Find out how many protons, neutrons, and electrons are. Learn about the number and arrangement of protons, neutrons, and electrons in iron, a common metal element with atomic number 26. Atoms of the same element that contain the same number of. Learn about the atomic number, mass number, and isotopes of atoms. Find out the stable and unstable isotopes of. Iron Neutrons.
From
Iron Neutrons Find out the stable and unstable isotopes of iron, and the most common alloy of iron, carbon steel. Atoms of the same element that contain the same number of. Find out how many protons, neutrons, and electrons are. Neutron number and mass number of iron. Learn about the atomic number, mass number, and isotopes of atoms. Learn about the atomic. Iron Neutrons.
From mokasinthebig.weebly.com
Iron atomic number mokasinthebig Iron Neutrons The chemical symbol for iron is fe. Neutron number and mass number of iron. Learn about the atomic number, mass number, and isotopes of atoms. The number of neutrons can be different, even in atoms of the same element. Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26 protons and variable. Iron Neutrons.
From www.slideserve.com
PPT Interaction of electrons with matter Interaction of photons with Iron Neutrons The number of neutrons can be different, even in atoms of the same element. Find out how many protons, neutrons, and electrons are. Learn about the atomic number, mass number, and isotopes of atoms. Learn about the location, charge, and relative mass of neutrons, the subatomic particles that act as nuclear glue in the nucleus. Learn about the atomic and. Iron Neutrons.
From anelementaday.wordpress.com
Day 3 Iron An Element A Day Iron Neutrons Find out how many protons, neutrons, and electrons are. Mass numbers of typical isotopes of iron are 56; Neutron number and mass number of iron. Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26 protons and variable number of neutrons. Learn about the atomic number, mass number, and isotopes of atoms.. Iron Neutrons.
From www.researchgate.net
Measured and calculated neutron spectra of the iron experiment Iron Neutrons Learn about the location, charge, and relative mass of neutrons, the subatomic particles that act as nuclear glue in the nucleus. Learn about the atomic number, mass number, and isotopes of atoms. The chemical symbol for iron is fe. The number of neutrons can be different, even in atoms of the same element. Mass numbers of typical isotopes of iron. Iron Neutrons.
From reviewhomedecor.co
Iron Periodic Table Protons Neutrons Electrons Review Home Decor Iron Neutrons Mass numbers of typical isotopes of iron are 56; Neutron number and mass number of iron. Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26 protons and variable number of neutrons. Find out how to calculate the mass number. The number of neutrons can be different, even in atoms of the. Iron Neutrons.
From
Iron Neutrons Mass numbers of typical isotopes of iron are 56; Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26 protons and variable number of neutrons. The chemical symbol for iron is fe. Learn about the number and arrangement of protons, neutrons, and electrons in iron, a common metal element with atomic number. Iron Neutrons.
From
Iron Neutrons Find out how to calculate the mass number. The number of neutrons can be different, even in atoms of the same element. Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26 protons and variable number of neutrons. The chemical symbol for iron is fe. Mass numbers of typical isotopes of iron. Iron Neutrons.
From
Iron Neutrons Atoms of the same element that contain the same number of. The chemical symbol for iron is fe. Learn about the location, charge, and relative mass of neutrons, the subatomic particles that act as nuclear glue in the nucleus. The number of neutrons can be different, even in atoms of the same element. Neutron number and mass number of iron.. Iron Neutrons.
From
Iron Neutrons The number of neutrons can be different, even in atoms of the same element. Find out the stable and unstable isotopes of iron, and the most common alloy of iron, carbon steel. Learn about the number and arrangement of protons, neutrons, and electrons in iron, a common metal element with atomic number 26. Atoms of the same element that contain. Iron Neutrons.
From utedzz.blogspot.com
Periodic Table Numbers Of Neutrons Protons And Electrons Periodic Iron Neutrons The number of neutrons can be different, even in atoms of the same element. Neutron number and mass number of iron. Learn about the location, charge, and relative mass of neutrons, the subatomic particles that act as nuclear glue in the nucleus. Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26. Iron Neutrons.
From www.bigstockphoto.com
3d Render Atom Structure Iron Image & Photo Bigstock Iron Neutrons Neutron number and mass number of iron. Learn about the atomic number, mass number, and isotopes of atoms. Mass numbers of typical isotopes of iron are 56; Atoms of the same element that contain the same number of. Find out the stable and unstable isotopes of iron, and the most common alloy of iron, carbon steel. Find out how to. Iron Neutrons.
From elchoroukhost.net
Iron Periodic Table Protons Neutrons And Electrons Elcho Table Iron Neutrons Atoms of the same element that contain the same number of. Mass numbers of typical isotopes of iron are 56; Find out how to calculate the mass number. Learn about the number and arrangement of protons, neutrons, and electrons in iron, a common metal element with atomic number 26. Find out how many protons, neutrons, and electrons are. The number. Iron Neutrons.
From reviewhomedecor.co
Iron Periodic Table Number Of Neutrons Review Home Decor Iron Neutrons Learn about the location, charge, and relative mass of neutrons, the subatomic particles that act as nuclear glue in the nucleus. Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26 protons and variable number of neutrons. The chemical symbol for iron is fe. Find out how to calculate the mass number.. Iron Neutrons.
From
Iron Neutrons The number of neutrons can be different, even in atoms of the same element. Learn about the location, charge, and relative mass of neutrons, the subatomic particles that act as nuclear glue in the nucleus. Learn about the atomic number, mass number, and isotopes of atoms. Find out how many protons, neutrons, and electrons are. Find out how to calculate. Iron Neutrons.
From sciencing.com
How to Find the Neutrons in the Periodic Table Sciencing Iron Neutrons Mass numbers of typical isotopes of iron are 56; Learn about the number and arrangement of protons, neutrons, and electrons in iron, a common metal element with atomic number 26. The number of neutrons can be different, even in atoms of the same element. Find out the stable and unstable isotopes of iron, and the most common alloy of iron,. Iron Neutrons.
From
Iron Neutrons Find out how many protons, neutrons, and electrons are. Find out how to calculate the mass number. The number of neutrons can be different, even in atoms of the same element. Learn about the number and arrangement of protons, neutrons, and electrons in iron, a common metal element with atomic number 26. Learn about the atomic and decay properties of. Iron Neutrons.
From ar.inspiredpencil.com
Periodic Table Of Elements With Protons Neutrons And Electrons Iron Neutrons Find out how many protons, neutrons, and electrons are. Learn about the number and arrangement of protons, neutrons, and electrons in iron, a common metal element with atomic number 26. Find out the stable and unstable isotopes of iron, and the most common alloy of iron, carbon steel. Learn about the atomic number, mass number, and isotopes of atoms. Learn. Iron Neutrons.
From
Iron Neutrons Find out the stable and unstable isotopes of iron, and the most common alloy of iron, carbon steel. Neutron number and mass number of iron. The chemical symbol for iron is fe. Atoms of the same element that contain the same number of. The number of neutrons can be different, even in atoms of the same element. Learn about the. Iron Neutrons.
From
Iron Neutrons The chemical symbol for iron is fe. Find out how many protons, neutrons, and electrons are. Learn about the location, charge, and relative mass of neutrons, the subatomic particles that act as nuclear glue in the nucleus. The number of neutrons can be different, even in atoms of the same element. Mass numbers of typical isotopes of iron are 56;. Iron Neutrons.
From
Iron Neutrons Find out how many protons, neutrons, and electrons are. The chemical symbol for iron is fe. Learn about the atomic number, mass number, and isotopes of atoms. The number of neutrons can be different, even in atoms of the same element. Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26 protons. Iron Neutrons.
From
Iron Neutrons The chemical symbol for iron is fe. Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26 protons and variable number of neutrons. Atoms of the same element that contain the same number of. The number of neutrons can be different, even in atoms of the same element. Mass numbers of typical. Iron Neutrons.
From
Iron Neutrons Find out the stable and unstable isotopes of iron, and the most common alloy of iron, carbon steel. Atoms of the same element that contain the same number of. Find out how many protons, neutrons, and electrons are. Learn about the number and arrangement of protons, neutrons, and electrons in iron, a common metal element with atomic number 26. The. Iron Neutrons.
From www.researchgate.net
C/E of peak neutron flux (3545 MeV) of the iron experiment for 40 MeV Iron Neutrons Learn about the atomic number, mass number, and isotopes of atoms. Learn about the location, charge, and relative mass of neutrons, the subatomic particles that act as nuclear glue in the nucleus. Find out how to calculate the mass number. The chemical symbol for iron is fe. Mass numbers of typical isotopes of iron are 56; The number of neutrons. Iron Neutrons.
From
Iron Neutrons Find out how many protons, neutrons, and electrons are. Learn about the number and arrangement of protons, neutrons, and electrons in iron, a common metal element with atomic number 26. Learn about the atomic and decay properties of all known isotopes of iron, a chemical element with 26 protons and variable number of neutrons. Find out the stable and unstable. Iron Neutrons.