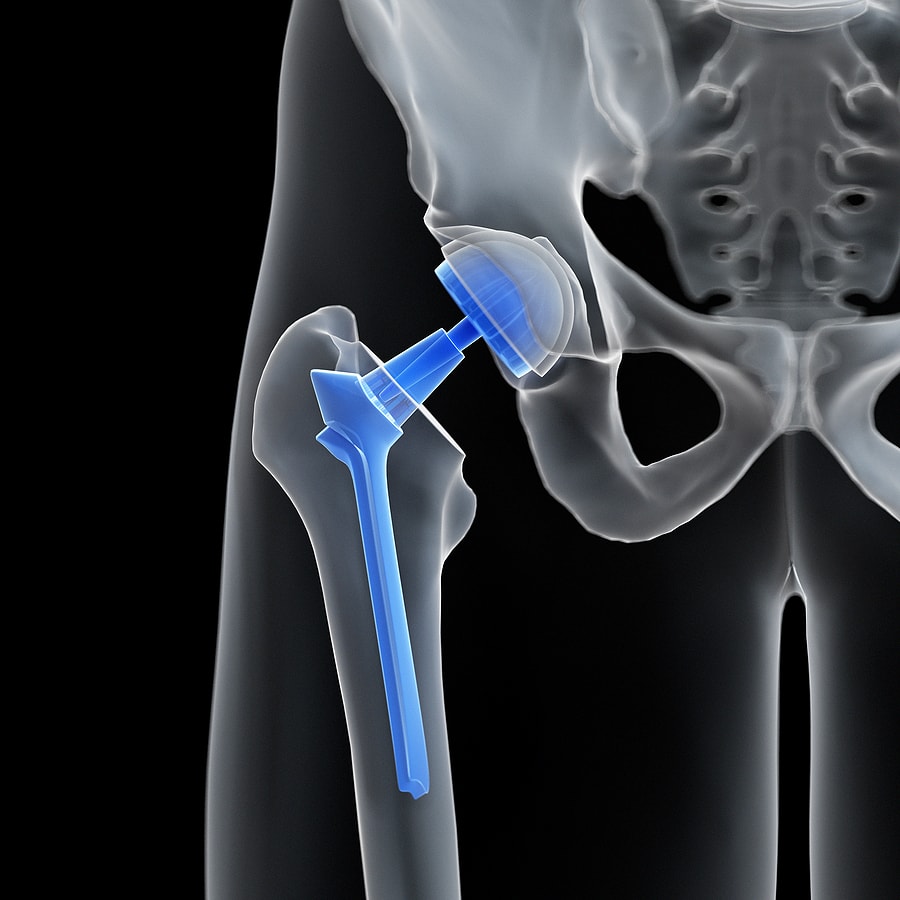
Zimmer Hip Replacement Recall . On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. The fda is getting the word out regarding a recall effort from zimmer biomet, related to its discontinued cpt hip implant and. Zimmer biomet will phase out sales of its cpt hip system by december due to concerns about the risk of thigh bone fractures,. In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk of. 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. Zimmer recalled its cpt hip system femoral stem 12/14 neck taper in july, updating the device’s instructions for use to. Zimmer biomet issued urgent medical device recall letter on 10/09/23 to distributors and risk managers via.
from www.dolmanlaw.com
The fda is getting the word out regarding a recall effort from zimmer biomet, related to its discontinued cpt hip implant and. 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. Zimmer recalled its cpt hip system femoral stem 12/14 neck taper in july, updating the device’s instructions for use to. On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. Zimmer biomet will phase out sales of its cpt hip system by december due to concerns about the risk of thigh bone fractures,. In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk of. Zimmer biomet issued urgent medical device recall letter on 10/09/23 to distributors and risk managers via.
Zimmer Hip Replacement Attorney in Florida
Zimmer Hip Replacement Recall Zimmer recalled its cpt hip system femoral stem 12/14 neck taper in july, updating the device’s instructions for use to. Zimmer recalled its cpt hip system femoral stem 12/14 neck taper in july, updating the device’s instructions for use to. On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. The fda is getting the word out regarding a recall effort from zimmer biomet, related to its discontinued cpt hip implant and. Zimmer biomet issued urgent medical device recall letter on 10/09/23 to distributors and risk managers via. Zimmer biomet will phase out sales of its cpt hip system by december due to concerns about the risk of thigh bone fractures,. In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk of.
From robertgardnerlaw.net
Hip or Knee Replacement Complications Robert Gardner Law Zimmer Hip Replacement Recall 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. Zimmer biomet issued urgent medical device recall letter on 10/09/23 to distributors and risk managers via. The fda is getting the word out regarding a recall effort from zimmer biomet, related to its discontinued cpt hip implant and. On august 25, 2022, the firm notified affected. Zimmer Hip Replacement Recall.
From www.classactionlawsuithelp.com
Zimmer Ordered to Pay 2 Million for Hip Replacement Complications Zimmer Hip Replacement Recall Zimmer recalled its cpt hip system femoral stem 12/14 neck taper in july, updating the device’s instructions for use to. In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk of. 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. Zimmer biomet. Zimmer Hip Replacement Recall.
From drugwatch.com
Hip Replacement Recall Causes and FDA Information Zimmer Hip Replacement Recall Zimmer biomet will phase out sales of its cpt hip system by december due to concerns about the risk of thigh bone fractures,. Zimmer biomet issued urgent medical device recall letter on 10/09/23 to distributors and risk managers via. Zimmer recalled its cpt hip system femoral stem 12/14 neck taper in july, updating the device’s instructions for use to. On. Zimmer Hip Replacement Recall.
From www.doccheckshop.eu
ErlerZimmer Hip implant model DocCheck Shop Zimmer Hip Replacement Recall Zimmer biomet will phase out sales of its cpt hip system by december due to concerns about the risk of thigh bone fractures,. On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased. Zimmer Hip Replacement Recall.
From giofdvjol.blob.core.windows.net
Zimmer Hip Replacement Lawsuit Settlement at Jenny Cottrell blog Zimmer Hip Replacement Recall On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk of. 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. The fda is getting the word out. Zimmer Hip Replacement Recall.
From www.alibaba.com
Zimmer Ceramic Hip Replacement Stainless Steel Orthopedics Veterinary Zimmer Hip Replacement Recall Zimmer biomet will phase out sales of its cpt hip system by december due to concerns about the risk of thigh bone fractures,. In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk of. 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to. Zimmer Hip Replacement Recall.
From www.cureus.com
Cureus Epidermolysis Bullosa A Case of Successful Total Hip Arthroplasty Zimmer Hip Replacement Recall 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. The fda is getting the word out regarding a recall effort from zimmer biomet, related to its discontinued cpt hip implant and. Zimmer biomet issued urgent medical device recall letter on 10/09/23 to distributors and risk managers via. Zimmer biomet will phase out sales of its. Zimmer Hip Replacement Recall.
From www.youtube.com
Zimmer Durom Cup Hip Implant Revision Surgery Help Plus My Personal Zimmer Hip Replacement Recall 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. The fda is getting the word out regarding a recall effort from zimmer biomet, related to its discontinued cpt hip implant and. Zimmer biomet issued urgent medical device recall letter on 10/09/23 to distributors and risk managers via. On august 25, 2022, the firm notified affected. Zimmer Hip Replacement Recall.
From www.groupactionlawyers.co.uk
Zimmer Biomet Hip & Trauma Instrument Recall Due To Risk Of Infection Zimmer Hip Replacement Recall On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. Zimmer recalled its cpt hip system femoral stem 12/14 neck taper in july, updating the device’s instructions for use to. 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. Zimmer biomet will phase out sales of its cpt hip system. Zimmer Hip Replacement Recall.
From www.enjuris.com
Zimmer Biomet Hip Replacement Devices, Failed Hip Implants Zimmer Hip Replacement Recall The fda is getting the word out regarding a recall effort from zimmer biomet, related to its discontinued cpt hip implant and. On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. Zimmer biomet issued urgent medical device recall letter on 10/09/23 to distributors and risk managers via. Zimmer biomet will phase out sales of. Zimmer Hip Replacement Recall.
From topclassactions.com
Zimmer Hip Implant Lawsuit Filed by Nevada Plaintiff Top Class Actions Zimmer Hip Replacement Recall The fda is getting the word out regarding a recall effort from zimmer biomet, related to its discontinued cpt hip implant and. On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk. Zimmer Hip Replacement Recall.
From www.zimmerbiomet.lat
Total Hip Arthroplasty Continuum® Acetabular System Zimmer Biomet Zimmer Hip Replacement Recall In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk of. On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. Zimmer recalled its cpt hip system femoral. Zimmer Hip Replacement Recall.
From attorneygroup.com
Texas Stryker Hip Recall Attorney Rejuvinate Hip Recall Lawyer Zimmer Hip Replacement Recall Zimmer recalled its cpt hip system femoral stem 12/14 neck taper in july, updating the device’s instructions for use to. 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. Zimmer biomet will phase out sales of its cpt hip system by december due to concerns about the risk of thigh bone fractures,. Zimmer biomet issued. Zimmer Hip Replacement Recall.
From www.youtube.com
Zimmer Hip & Knee Replacement Embroiled In Metallosis Lawsuits Zimmer Hip Replacement Recall Zimmer recalled its cpt hip system femoral stem 12/14 neck taper in july, updating the device’s instructions for use to. In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk of. The fda is getting the word out regarding a recall effort from zimmer biomet, related to. Zimmer Hip Replacement Recall.
From www.dolmanlaw.com
Zimmer Hip Replacement Attorney in Florida Zimmer Hip Replacement Recall Zimmer recalled its cpt hip system femoral stem 12/14 neck taper in july, updating the device’s instructions for use to. On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. Zimmer biomet will phase out sales of its cpt hip system by december due to concerns about the risk of thigh bone fractures,. 2/7/23 zimmer. Zimmer Hip Replacement Recall.
From www.slideshare.net
Hip Recall Lawyer Zimmer Hip Replacement Recall Zimmer biomet issued urgent medical device recall letter on 10/09/23 to distributors and risk managers via. In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk of. 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. The fda is getting the word. Zimmer Hip Replacement Recall.
From world-at-your-fingertips-curriculum.blogspot.com
hip replacement recall pictures worldatyourfingertipscurriculum Zimmer Hip Replacement Recall Zimmer biomet issued urgent medical device recall letter on 10/09/23 to distributors and risk managers via. The fda is getting the word out regarding a recall effort from zimmer biomet, related to its discontinued cpt hip implant and. Zimmer biomet will phase out sales of its cpt hip system by december due to concerns about the risk of thigh bone. Zimmer Hip Replacement Recall.
From www.youtube.com
Stryker Hip Recall Info Litigation Timeline YouTube Zimmer Hip Replacement Recall 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. Zimmer recalled its cpt hip system femoral stem 12/14 neck taper in july, updating the device’s instructions for use to. In early july, zimmer biomet initiated a recall to update instructions. Zimmer Hip Replacement Recall.
From kog.net.au
Total Hip Replacement Orthopaedic Surgeon In Wantrina, Melbourne Zimmer Hip Replacement Recall Zimmer biomet issued urgent medical device recall letter on 10/09/23 to distributors and risk managers via. On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. In early july, zimmer biomet initiated a recall to update instructions for use for the. Zimmer Hip Replacement Recall.
From nashfranciskato.com
FDA Recall Issued Zimmer M/L Taper Hip Prosthesis » Nash and Zimmer Hip Replacement Recall Zimmer biomet will phase out sales of its cpt hip system by december due to concerns about the risk of thigh bone fractures,. In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk of. 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to. Zimmer Hip Replacement Recall.
From giofdvjol.blob.core.windows.net
Zimmer Hip Replacement Lawsuit Settlement at Jenny Cottrell blog Zimmer Hip Replacement Recall On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. Zimmer biomet will phase out sales of its cpt hip system by december due to concerns about the risk of thigh bone fractures,. The fda is getting the word out regarding a recall effort from zimmer biomet, related to its discontinued cpt hip implant and.. Zimmer Hip Replacement Recall.
From topclassactions.com
Zimmer Versys Hip Replacement is Defective, Says Patient Zimmer Hip Replacement Recall Zimmer biomet issued urgent medical device recall letter on 10/09/23 to distributors and risk managers via. In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk of. On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. Zimmer recalled its cpt hip. Zimmer Hip Replacement Recall.
From sheller.com
DEPUY ASR XL Hip Implant Sheller, P.C. Law Firm Zimmer Hip Replacement Recall Zimmer biomet issued urgent medical device recall letter on 10/09/23 to distributors and risk managers via. 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. The fda is getting the word out regarding a recall effort from zimmer biomet, related to its discontinued cpt hip implant and. On august 25, 2022, the firm notified affected. Zimmer Hip Replacement Recall.
From implantidentifier.app
Implant Identifier Zimmer Hip Replacement Recall 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. Zimmer recalled its cpt hip system femoral stem 12/14 neck taper in july, updating the device’s instructions for use to. In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk of. Zimmer biomet. Zimmer Hip Replacement Recall.
From www.arthritis-health.com
Zimmer Biomet Hip Replacement Zimmer Hip Replacement Recall In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk of. Zimmer biomet issued urgent medical device recall letter on 10/09/23 to distributors and risk managers via. Zimmer recalled its cpt hip system femoral stem 12/14 neck taper in july, updating the device’s instructions for use to.. Zimmer Hip Replacement Recall.
From www.drugdangers.com
Zimmer Hip Replacement Recall Durom Cup Recall Zimmer Hip Replacement Recall 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. The fda is getting the word out regarding a recall effort from zimmer biomet, related to its discontinued cpt hip implant and. On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. Zimmer biomet issued urgent medical device recall letter on. Zimmer Hip Replacement Recall.
From www.lawsuit-information-center.com
Zimmer Hip Replacement Recall Lawsuits — Lawsuit Information Center Zimmer Hip Replacement Recall In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk of. On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. The fda is getting the word out. Zimmer Hip Replacement Recall.
From www.laorthosurgeon.com
Hip Replacement ADAM SASSOON M.D., M.S. Zimmer Hip Replacement Recall Zimmer biomet will phase out sales of its cpt hip system by december due to concerns about the risk of thigh bone fractures,. Zimmer biomet issued urgent medical device recall letter on 10/09/23 to distributors and risk managers via. In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an. Zimmer Hip Replacement Recall.
From dokumen.tips
(PDF) IMAGE TO COME Zimmer Biometprod® LD/Fx Cemented and PressFit Zimmer Hip Replacement Recall Zimmer biomet issued urgent medical device recall letter on 10/09/23 to distributors and risk managers via. In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk of. 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. Zimmer recalled its cpt hip system. Zimmer Hip Replacement Recall.
From www.drugdangers.com
Hip Replacement Recall Recall Process & Causes Zimmer Hip Replacement Recall 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. Zimmer biomet will phase out sales of its cpt hip system by december due to concerns about the risk of thigh bone fractures,. Zimmer biomet issued urgent medical device recall letter on 10/09/23 to distributors and risk managers via. On august 25, 2022, the firm notified. Zimmer Hip Replacement Recall.
From mcintyrelaw.com
Zimmer Hip Replacement Lawsuits McIntyre Law Zimmer Hip Replacement Recall 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. Zimmer biomet will phase out sales of its cpt hip system by december due to concerns about the risk of thigh bone fractures,. Zimmer recalled its cpt hip system femoral stem 12/14 neck taper in july, updating the device’s instructions for use to. Zimmer biomet issued. Zimmer Hip Replacement Recall.
From www.druganddevicewatch.com
Zimmer VerSys Hip Implant Recalled Drug And Device Watch Zimmer Hip Replacement Recall On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. The fda is getting the word out regarding a recall effort from zimmer biomet, related to its discontinued cpt hip implant and. Zimmer recalled its cpt hip system femoral stem 12/14. Zimmer Hip Replacement Recall.
From www.youtube.com
Hip Animation from Zimmer Biomet YouTube Zimmer Hip Replacement Recall On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk of. The fda is getting the word out regarding a recall effort from zimmer biomet, related to its discontinued cpt hip implant. Zimmer Hip Replacement Recall.
From www.zimmerbiomet.com
Avenir Hip Complete System Total Hip Arthroplasty Zimmer Biomet Zimmer Hip Replacement Recall Zimmer recalled its cpt hip system femoral stem 12/14 neck taper in july, updating the device’s instructions for use to. On august 25, 2022, the firm notified affected customers via urgent medical device recall letters. 2/7/23 zimmer biomet issued urgent medical device recall expansion letter on 2/07/23 to distributors,. Zimmer biomet will phase out sales of its cpt hip system. Zimmer Hip Replacement Recall.
From www.zimmerbiomet.com
Total Hip Arthroplasty Taperloc Complete Hip System Zimmer Biomet Zimmer Hip Replacement Recall In early july, zimmer biomet initiated a recall to update instructions for use for the cpt hip system due to an increased risk of. Zimmer biomet will phase out sales of its cpt hip system by december due to concerns about the risk of thigh bone fractures,. On august 25, 2022, the firm notified affected customers via urgent medical device. Zimmer Hip Replacement Recall.